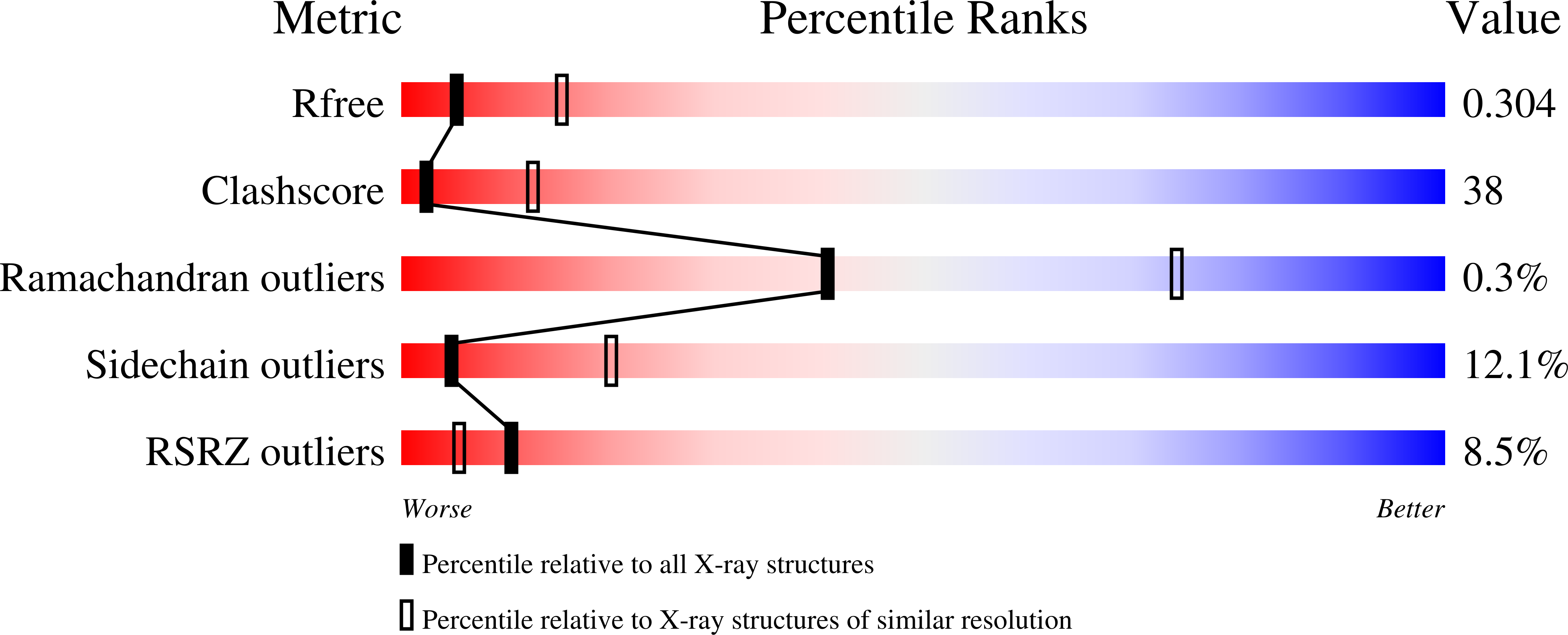
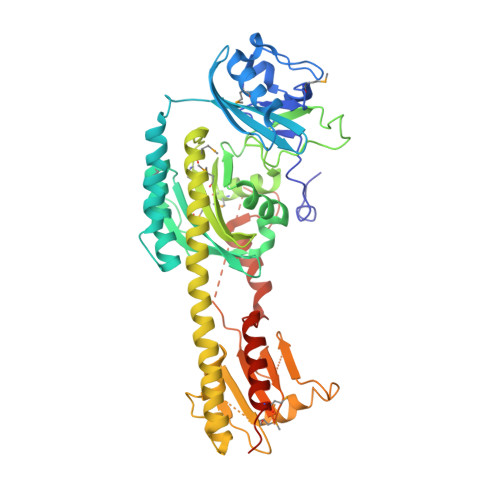
Conformational differences between the Pfr and Pr states in Pseudomonas aeruginosa bacteriophytochrome.
Yang, X., Kuk, J., Moffat, K.(2009) Proc Natl Acad Sci U S A 106: 15639-15644
- PubMed: 19720999
- DOI: https://doi.org/10.1073/pnas.0902178106
- Primary Citation of Related Structures:
3G6O, 3IBR - PubMed Abstract:
Phytochromes are red-light photoreceptors that regulate light responses in plants, fungi, and bacteria by means of reversible photoconversion between red (Pr) and far-red (Pfr) light-absorbing states. Here, we report the crystal structure of the Q188L mutant of Pseudomonas aeruginosa bacteriophytochrome (PaBphP) photosensory core module, which exhibits altered photoconversion behavior and different crystal packing from wild type. We observe two distinct chromophore conformations in the Q188L crystal structure that we identify with the Pfr and Pr states. The Pr/Pfr compositions, varying from crystal to crystal, seem to correlate with light conditions under which the Q188L crystals are cryoprotected. We also compare all known Pr and Pfr structures. Using site-directed mutagenesis, we identify residues that are involved in stabilizing the 15Ea (Pfr) and 15Za (Pr) configurations of the biliverdin chromophore. Specifically, Ser-261 appears to be essential to form a stable Pr state in PaBphP, possibly by means of its interaction with the propionate group of ring C. We propose a "flip-and-rotate" model that summarizes the major conformational differences between the Pr and Pfr states of the chromophore and its binding pocket.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Chicago, 929 East 57th Street, Chicago, IL 60637, USA. [email protected]