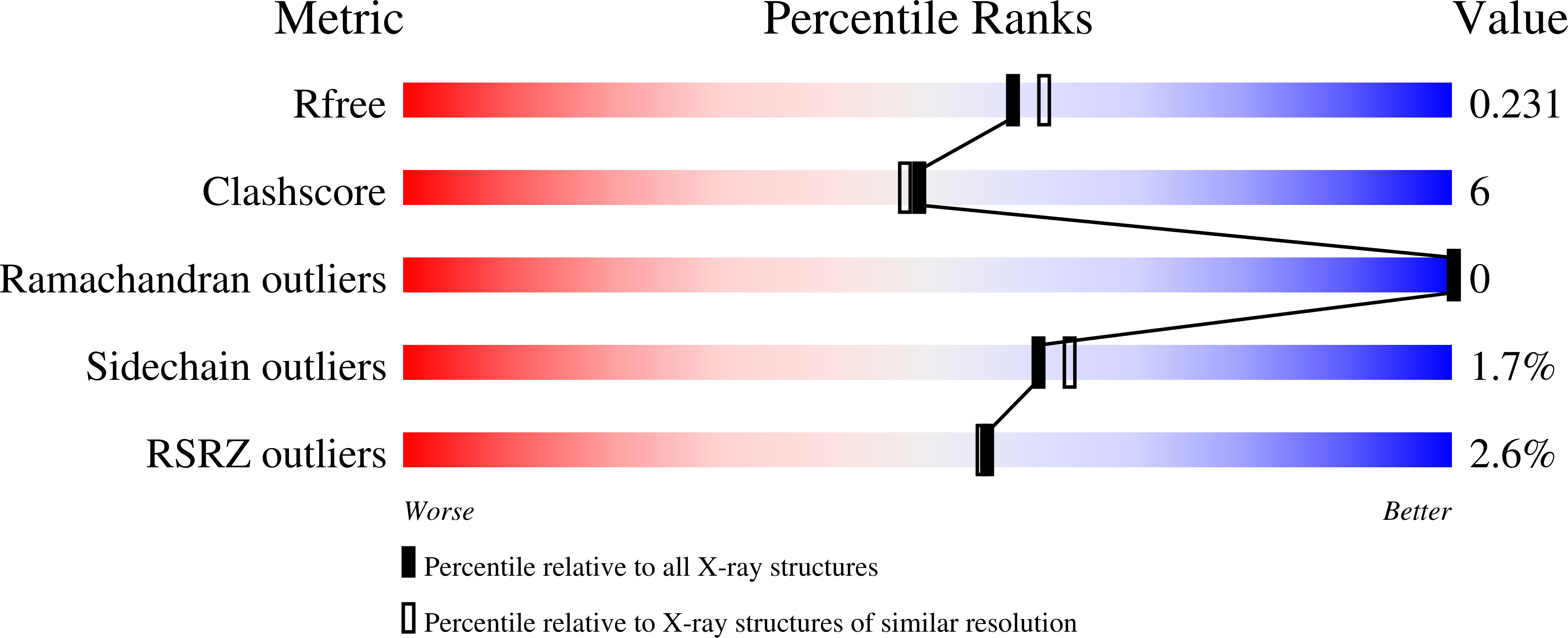
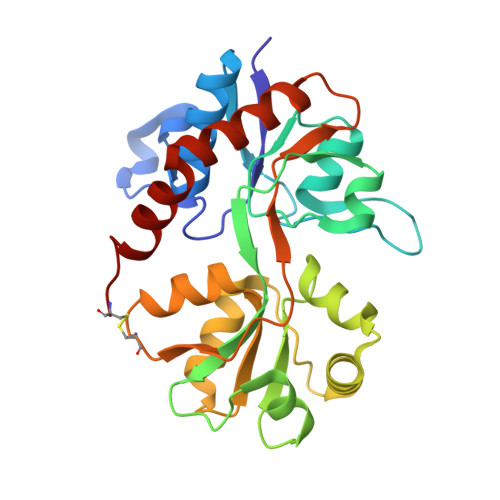
Thermodynamics and mechanism of the interaction of willardiine partial agonists with a glutamate receptor: implications for drug development.
Martinez, M., Ahmed, A.H., Loh, A.P., Oswald, R.E.(2014) Biochemistry 53: 3790-3795
- PubMed: 24850223
- DOI: https://doi.org/10.1021/bi500511m
- Primary Citation of Related Structures:
4Q30 - PubMed Abstract:
Understanding the thermodynamics of binding of a lead compound to a receptor can provide valuable information for drug design. The binding of compounds, particularly partial agonists, to subtypes of the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor is, in some cases, driven by increases in entropy. Using a series of partial agonists based on the structure of the natural product, willardiine, we show that the charged state of the ligand determines the enthalpic contribution to binding. Willardiines have uracil rings with pKa values ranging from 5.5 to 10. The binding of the charged form is largely driven by enthalpy, while that of the uncharged form is largely driven by entropy. This is due at least in part to changes in the hydrogen bonding network within the binding site involving one water molecule. This work illustrates the importance of charge to the thermodynamics of binding of agonists and antagonists to AMPA receptors and provides clues for further drug discovery.
Organizational Affiliation:
Department of Molecular Medicine, Cornell University , Ithaca, New York 14853, United States.