Fast Kinetics Reveals Rate-Limiting Oxidation and the Role of the Aromatic Cage in the Mechanism of the Nicotine-Degrading Enzyme NicA2.
Tararina, M.A., Dam, K.K., Dhingra, M., Janda, K.D., Palfey, B.A., Allen, K.N.(2021) Biochemistry 60: 259-273
- PubMed: 33464876
- DOI: https://doi.org/10.1021/acs.biochem.0c00855
- Primary Citation of Related Structures:
7KHN, 7KHO - PubMed Abstract:
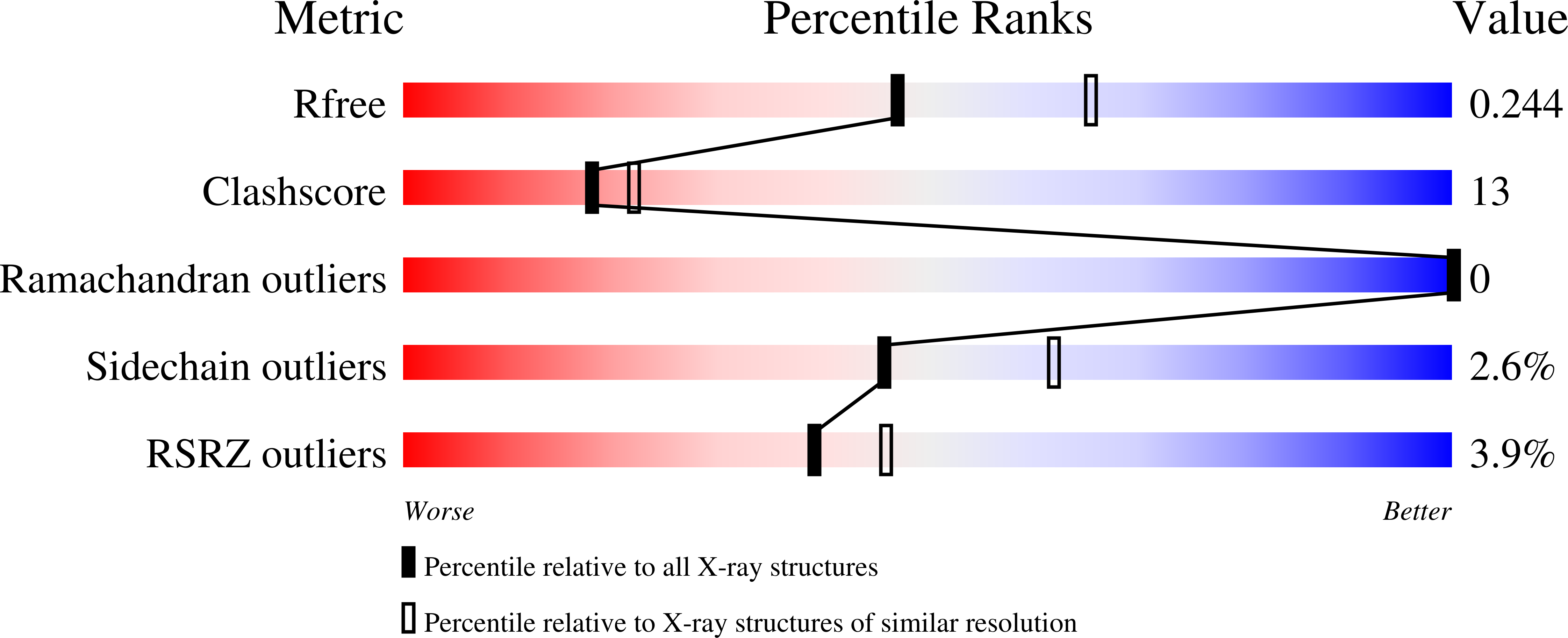
In Pseudomonas putida , the flavoprotein nicotine oxidoreductase (NicA2) catalyzes the oxidation of ( S )-nicotine to N -methyl-myosmine, which is nonenzymatically hydrolyzed to pseudooxynicotine. Structural analysis reveals a monoamine oxidase (MAO)-like fold with a conserved FAD-binding domain and variable substrate-binding domain. The flavoenzyme has a unique variation of the classic aromatic cage with flanking residue pair W427/N462. Previous mechanistic studies using O 2 as the oxidizing substrate show that NicA2 has a low apparent K m of 114 nM for ( S )-nicotine with a very low apparent turnover number ( k cat of 0.006 s -1 ). Herein, the mechanism of NicA2 was analyzed by transient kinetics. Single-site variants of W427 and N462 were used to probe the roles of these residues. Although several variants had moderately higher oxidase activity (7-12-fold), their reductive half-reactions using ( S )-nicotine were generally significantly slower than that of wild-type NicA2. Notably, the reductive half-reaction of wild-type NicA2 is 5 orders of magnitude faster than the oxidative half-reaction with an apparent pseudo-first-order rate constant for the reaction of oxygen similar to k cat . X-ray crystal structures of the N462V and N462Y/W427Y variants complexed with ( S )-nicotine (at 2.7 and 2.3 Å resolution, respectively) revealed no significant active-site rearrangements. A second substrate-binding site was identified in N462Y/W427Y, consistent with observed substrate inhibition. Together, these findings elucidate the mechanism of a flavoenzyme that preferentially oxidizes tertiary amines with an efficient reductive half-reaction and a very slow oxidative half-reaction when O 2 is the oxidizing substrate, suggesting that the true oxidizing agent is unknown.
Organizational Affiliation:
Program in Biomolecular Pharmacology, Boston University School of Medicine, 72 East Concord Street, Boston, Massachusetts 02118, United States.