Crystal structure of Calotropain FI from Calotropis gigantea
Kumar, A., Jamdar, S.N., Srivastava, G., Makde, R.D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Procerain | 214 | Calotropis gigantea | Mutation(s): 0 | 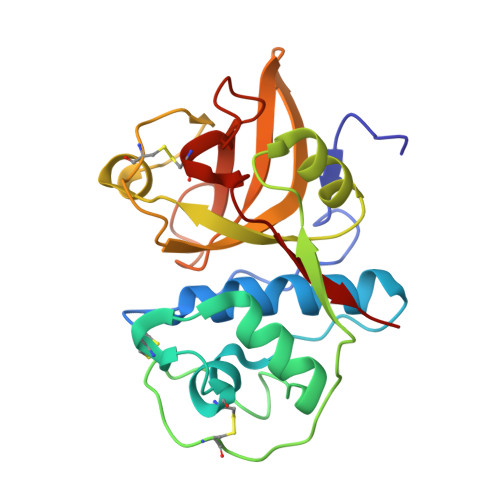 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| E64 (Subject of Investigation/LOI) Query on E64 | B [auth A] | N-[N-[1-HYDROXYCARBOXYETHYL-CARBONYL]LEUCYLAMINO-BUTYL]-GUANIDINE C15 H30 N5 O5 QPQNJAXBPHVASB-QWRGUYRKSA-O |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CME Query on CME | A | L-PEPTIDE LINKING | C5 H11 N O3 S2 |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 68.616 | α = 90 |
| b = 121.272 | β = 90 |
| c = 61.006 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHENIX | phasing |
| RESOLVE | model building |
| Coot | model building |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other government | India | -- |