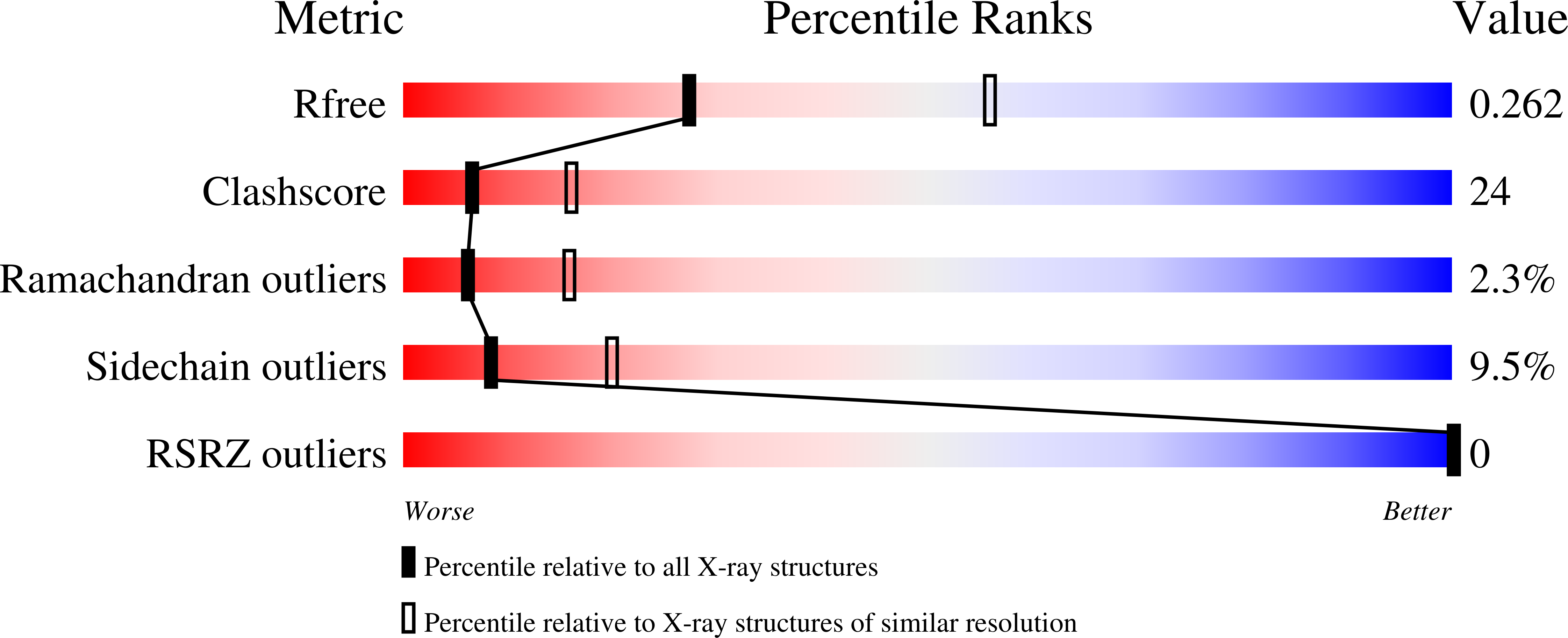
Crystal structure of a plant catechol oxidase containing a dicopper center.
Klabunde, T., Eicken, C., Sacchettini, J.C., Krebs, B.(1998) Nat Struct Biol 5: 1084-1090
- PubMed: 9846879
- DOI: https://doi.org/10.1038/4193
- Primary Citation of Related Structures:
1BT1, 1BT2, 1BT3, 1BUG - PubMed Abstract:
Catechol oxidases are ubiquitous plant enzymes containing a dinuclear copper center. In the wound-response mechanism of the plant they catalyze the oxidation of a broad range of ortho-diphenols to the corresponding o-quinones coupled with the reduction of oxygen to water. The crystal structures of the enzyme from sweet potato in the resting dicupric Cu(II)-Cu(II) state, the reduced dicuprous Cu(I)-Cu(I) form, and in complex with the inhibitor phenylthiourea were analyzed. The catalytic copper center is accommodated in a central four-helix-bundle located in a hydrophobic pocket close to the surface. Both metal binding sites are composed of three histidine ligands. His 109, ligated to the CuA site, is covalently linked to Cys 92 by an unusual thioether bond. Based on biochemical, spectroscopic and the presented structural data, a catalytical mechanism is proposed in which one of the oxygen atoms of the diphenolic substrate binds to CuB of the oxygenated enzyme.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Texas A&M University, College Station 77843-2128, USA.