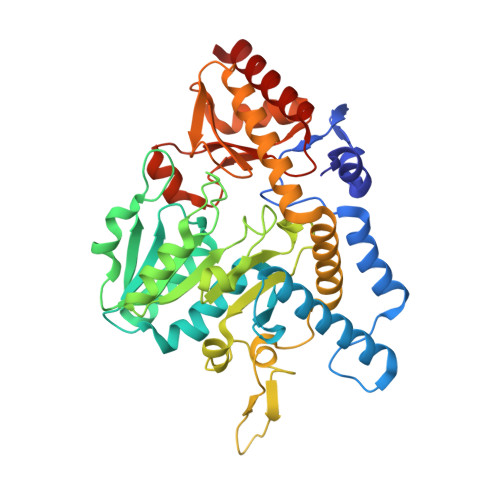
Analysis of the E. coli NifS CsdB Protein at 2.0A Reveals the Structural Basis for Perselenide and Persulfide Intermediate Formation
Lima, C.D.(2002) J Mol Biol 315: 1199-1208
- PubMed: 11827487
- DOI: https://doi.org/10.1006/jmbi.2001.5308
- Primary Citation of Related Structures:
1JF9, 1KMJ, 1KMK - PubMed Abstract:
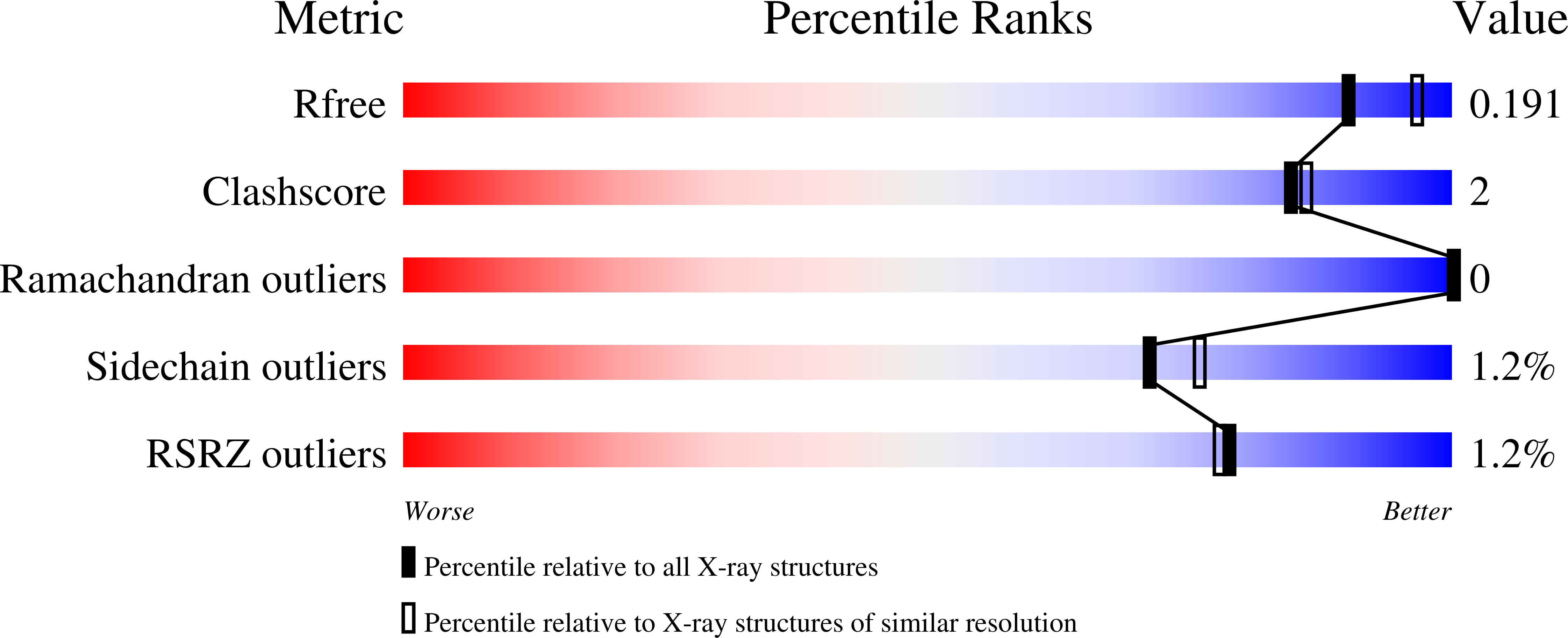
The Escherichia coli NifS CsdB protein is a member of the homodimeric pyridoxal 5'-phosphate (PLP)-dependent family of enzymes. These enzymes are capable of decomposing cysteine or selenocysteine into L-alanine and sulfur or selenium, respectively. E. coli NifS CsdB has a high specificity for L-selenocysteine in comparison to l-cysteine, suggesting a role for this enzyme is selenium metabolism. The 2.0 A crystal structure of E. coli NifS CsdB reveals a high-resolution view of the active site of this enzyme in apo-, persulfide, perselenide, and selenocysteine-bound intermediates, suggesting a mechanism for the stabilization of the enzyme persulfide and perselenide intermediates during catalysis, a necessary intermediate in the formation of sulfur and selenium containing metabolites.
Organizational Affiliation:
Biochemistry Department and Structural Biology Program, Weill Medical College of Cornell University, New York, NY 10021, USA. [email protected]