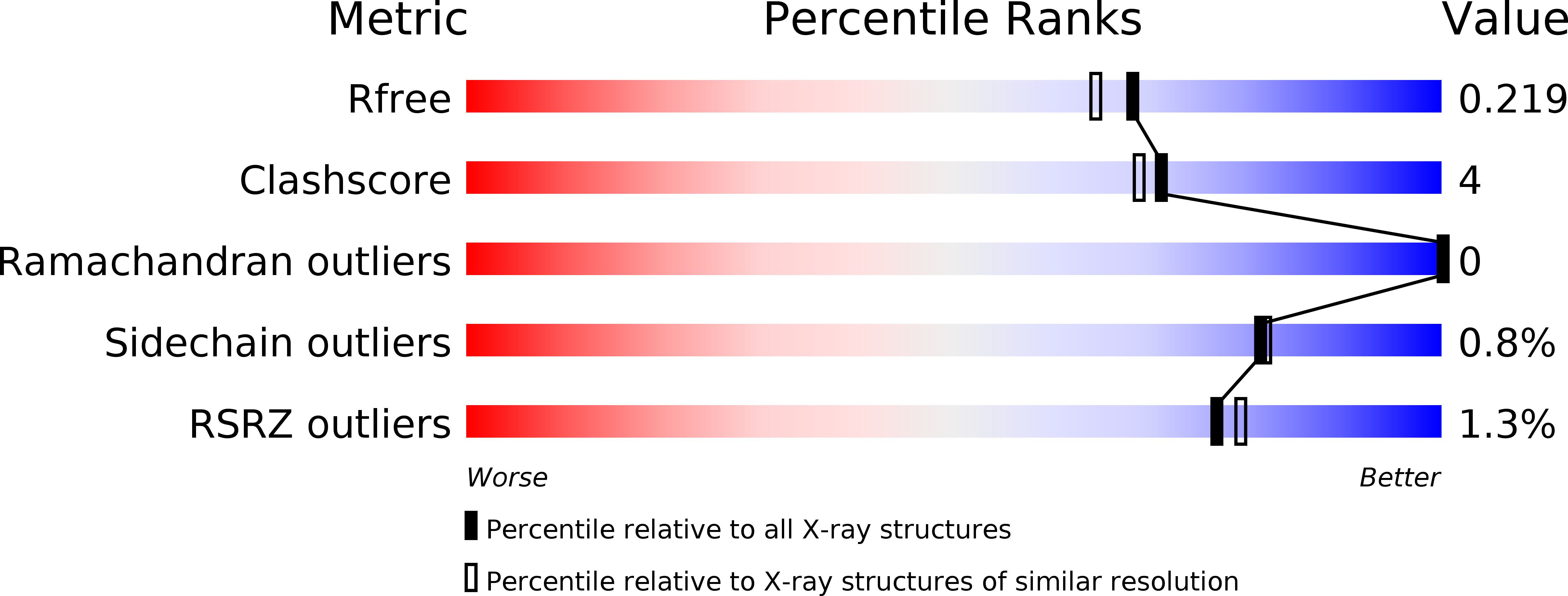
Structure of methylene-tetrahydromethanopterin dehydrogenase from methylobacterium extorquens AM1.
Ermler, U., Hagemeier, C.H., Roth, A., Demmer, U., Grabarse, W., Warkentin, E., Vorholt, J.A.(2002) Structure 10: 1127-1137
- PubMed: 12176390
- DOI: https://doi.org/10.1016/s0969-2126(02)00802-x
- Primary Citation of Related Structures:
1LU9, 1LUA - PubMed Abstract:
NADP-dependent methylene-H(4)MPT dehydrogenase, MtdA, from Methylobacterium extorquens AM1 catalyzes the dehydrogenation of methylene-tetrahydromethanopterin and methylene-tetrahydrofolate with NADP(+) as cosubstrate. The X-ray structure of MtdA with and without NADP bound was established at 1.9 A resolution. The enzyme is present as a homotrimer. The alpha,beta fold of the monomer is related to that of methylene-H(4)F dehydrogenases, suggesting a common evolutionary origin. The position of the active site is located within a large crevice built up by the two domains of one subunit and one domain of a second subunit. Methylene-H(4)MPT could be modeled into the cleft, and crucial active site residues such as Phe18, Lys256, His260, and Thr102 were identified. The molecular basis of the different substrate specificities and different catalytic demands of MtdA compared to methylene-H(4)F dehydrogenases are discussed.
Organizational Affiliation:
Max-Planck-Institut für Biophysik, Heinrich-Hoffmann-Strasse 7, D-60528 Frankfurt am Main, Germany. [email protected]