The structure of a mutant enzyme of Coprinus cinereus peroxidase provides an understanding of its increased thermostability.
Houborg, K., Harris, P., Poulsen, J.C., Schneider, P., Svendsen, A., Larsen, S.(2003) Acta Crystallogr D Biol Crystallogr 59: 997-1003
- PubMed: 12777761
- DOI: https://doi.org/10.1107/s0907444903006784
- Primary Citation of Related Structures:
1LY8 - PubMed Abstract:
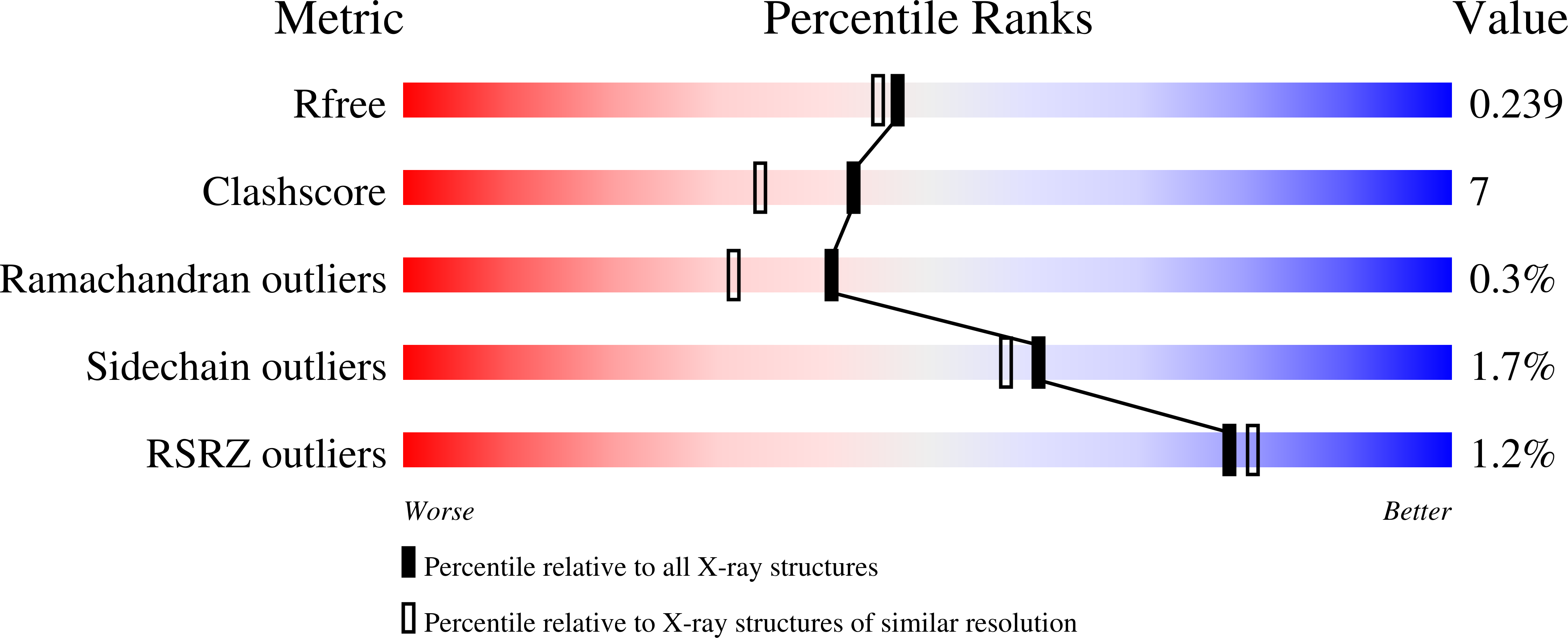
Seven amino-acid substitutions introduced into the 343 amino-acid-long sequence of Coprinus cinereus peroxidase (CiP) led to a mutant enzyme (TS-rCiP) which is more stable than the native enzyme at higher temperature, pH and hydrogen peroxide concentrations. It is therefore more suitable for industrial applications. A structure determination was conducted on a deglycosylated but still active form of TS-rCiP based on X-ray diffraction data to 2.05 A resolution measured on a crystal cooled to 100 K and refined to R = 0.202 and R(free) = 0.249. The increased stability of the TS-rCiP enzyme can be understood from the structural changes of the TS-rCiP structure revealed by a comparative analysis with other known CiP structures. One of the more significant changes caused by three of the substitutions, I49S, V53A and T121A, is the conversion of a hydrophobic pocket into a hydrophilic pocket with associated changes in the water structure and the hydrogen-bonding interactions. The E239G substitution, which gives rise to increased thermostability at high pH, creates changes in the water structure and in the orientation of a phenylalanine (Phe236) in its vicinity. The three substitutions M166F, M242 and Y242F introduced to increase the oxidative stability do not introduce any structural changes.
Organizational Affiliation:
Centre for Crystallographic Studies, University of Copenhagen, Universitetsparken 5, 2100 Copenhagen, Denmark.