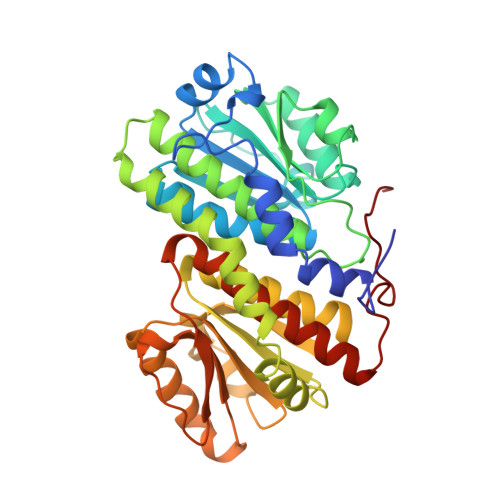
Involvement of the C terminus in intramolecular nitrogen channeling in glucosamine 6-phosphate synthase: evidence from a 1.6 A crystal structure of the isomerase domain.
Teplyakov, A., Obmolova, G., Badet-Denisot, M.A., Badet, B., Polikarpov, I.(1998) Structure 6: 1047-1055
- PubMed: 9739095
- DOI: https://doi.org/10.1016/s0969-2126(98)00105-1
- Primary Citation of Related Structures:
1MOQ - PubMed Abstract:
Glucosamine 6-phosphate synthase (GlmS) catalyses the first step in hexosamine metabolism, converting fructose-6P (6 phosphate) into glucosamine-6P using glutamine as a nitrogen source. GlmS is a bienzyme complex consisting of two domains that catalyse glutamine hydrolysis and sugar-phosphate isomerisation, respectively. Knowledge of the three-dimensional structure of GlmS is essential for understanding the general principles of catalysis by ketol isomerases and the mechanism of nitrogen transfer in glutamine amidotransferases. The crystal structure of the isomerase domain of the Escherichia coli GlmS with the reaction product, glucosamine-6P, has been determined at 1.57 A resolution. It is comprised of two topologically identical subdomains, each of which is dominated by a nucleotide-binding motif of a flavodoxin type. The catalytic site is assembled by dimerisation of the protein. The isomerase active site of GlmS seems to be the result of evolution through gene duplication and subsequent dimerisation. Isomerisation of fructose-6P is likely to involve the formation of a Schiff base with Lys603 of the enzyme, the ring-opening step catalysed by His504, and the proton transfer from C1 to C2 of the substrate effected by Glu488. The highly conserved C-terminal fragment of the chain may play a key role in substrate binding, catalysis and communication with the glutaminase domain. The corresponding sequence pattern DXPXXLAK[SC]VT (in single-letter amino-acid code, where X is any amino acid and letters in brackets indicate that either serine or cysteine may take this position) may be considered as a fingerprint of GlmS.
Organizational Affiliation:
European Molecular Biology Laboratory, Germany. [email protected]