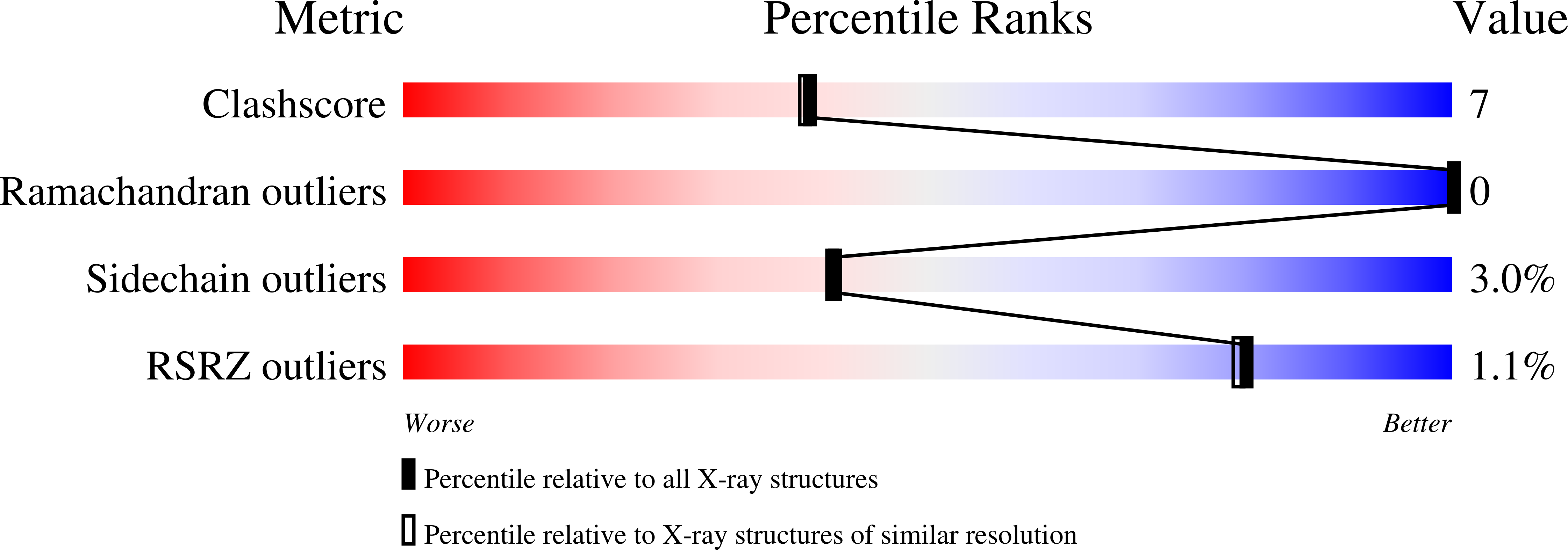
The mechanism of sugar phosphate isomerization by glucosamine 6-phosphate synthase.
Teplyakov, A., Obmolova, G., Badet-Denisot, M.A., Badet, B.(1999) Protein Sci 8: 596-602
- PubMed: 10091662
- DOI: https://doi.org/10.1110/ps.8.3.596
- Primary Citation of Related Structures:
1MOR, 1MOS - PubMed Abstract:
Glucosamine 6-phosphate synthase converts fructose-6P into glucosamine-6P or glucose-6P depending on the presence or absence of glutamine. The isomerase activity is associated with a 40-kDa C-terminal domain, which has already been characterized crystallographically. Now the three-dimensional structures of the complexes with the reaction product glucose-6P and with the transition state analog 2-amino-2-deoxyglucitol-6P have been determined. Glucose-6P binds in a cyclic form whereas 2-amino-2-deoxyglucitol-6P is in an extended conformation. The information on ligand-protein interactions observed in the crystal structures together with the isotope exchange and site-directed mutagenesis data allow us to propose a mechanism of the isomerase activity of glucosamine-6P synthase. The sugar phosphate isomerization involves a ring opening step catalyzed by His504 and an enolization step with Glu488 catalyzing the hydrogen transfer from C1 to C2 of the substrate. The enediol intermediate is stabilized by a helix dipole and the epsilon-amino group of Lys603. Lys485 may play a role in deprotonating the hydroxyl O1 of the intermediate.
Organizational Affiliation:
Genetics and Biochemistry Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland 20892, USA. [email protected]