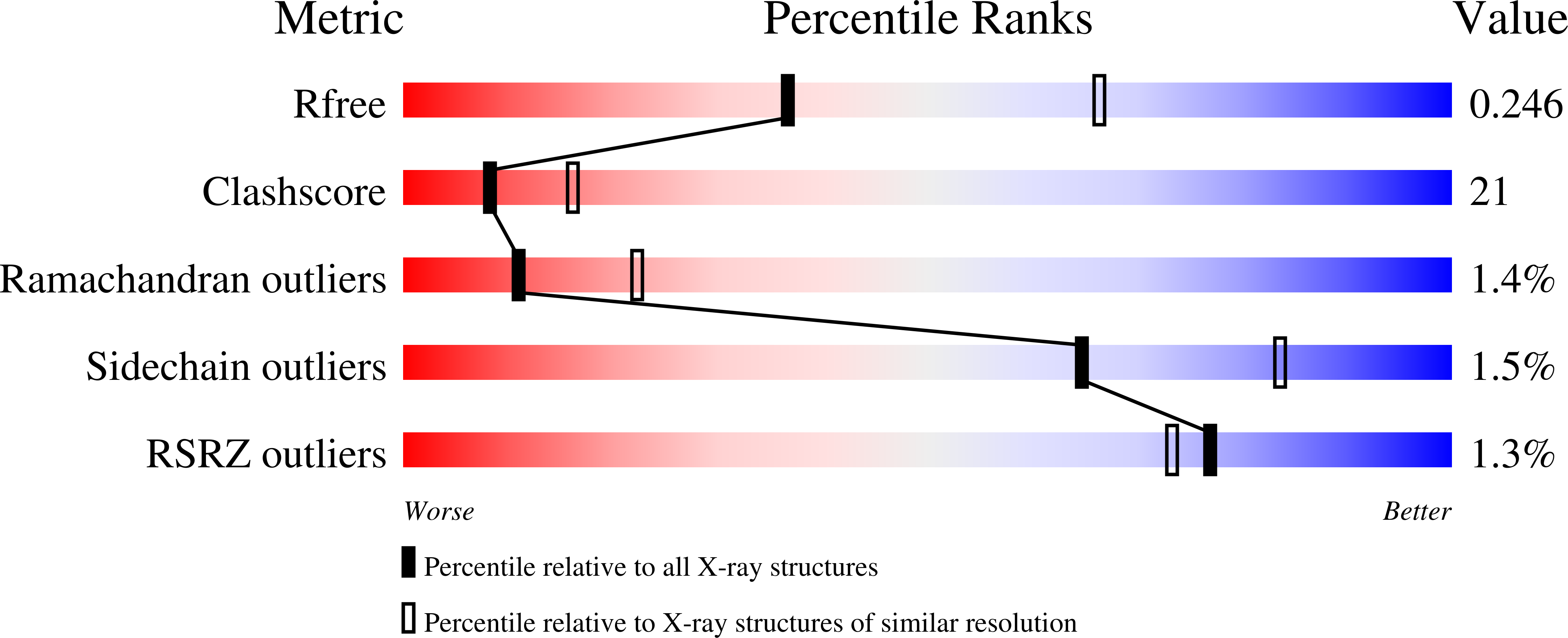
Substrate Binding and Catalysis of Ecto-Adp-Ribosyltransferase 2.2 From Rat
Ritter, H., Koch-Nolte, F., Marquez, V.E., Schulz, G.E.(2003) Biochemistry 42: 10155
- PubMed: 12939142
- DOI: https://doi.org/10.1021/bi034625w
- Primary Citation of Related Structures:
1OG1, 1OG3, 1OG4 - PubMed Abstract:
The structures of beta-methylenethiazole-4-carboxamide adenine dinucleotide (TAD), NAD(+), and NADH as bound to ecto-ADP-ribosyltransferase 2.2 from rat and to its mutants E189I and E189A, respectively, have been established. The positions and conformations of NAD(+) and its analogues agree in general with those in other ADP-ribosyltransferases. The kinetic constants for NAD(+) hydrolysis were determined by RP-HPLC. The specific activity amounts to 26 units/mg, which is 6000-fold higher than a previously reported rate and 500-fold higher than the hydrolysis rates of other ADP-ribosyltransferases, confirming that hydrolysis is the major function of this enzyme. On the basis of structures and mutant activities, a catalytic mechanism is proposed. The known auto-ADP-ribosylation of the enzyme at the suggested position R184 is supported by one of the crystal structures where the nucleophile position is occupied by an Neta atom of this arginine which in turn is backed up by the base E159.
Organizational Affiliation:
Institut für Organische Chemie und Biochemie, Albert-Ludwigs-Universität, Albertstrasse 21, Freiburg im Breisgau 79104, Germany.