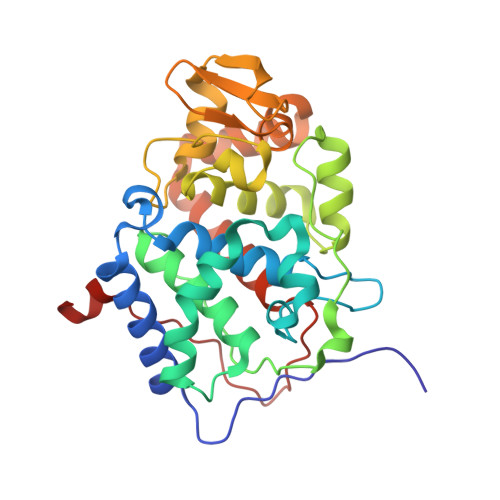
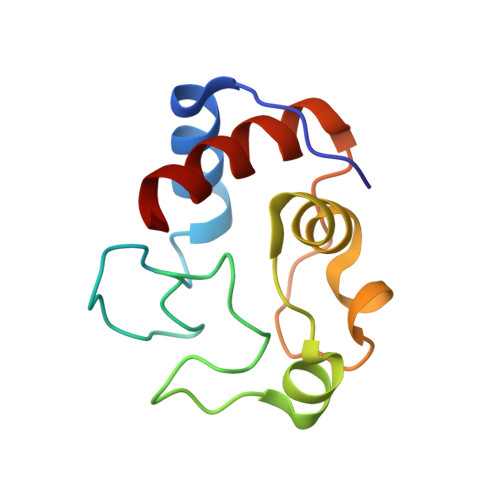
Crystal structure and characterization of a cytochrome c peroxidase-cytochrome c site-specific cross-link
Guo, M., Bhaskar, B., Li, H., Barrows, T.P., Poulos, T.L.(2004) Proc Natl Acad Sci U S A 101: 5940-5945
- PubMed: 15071191
- DOI: https://doi.org/10.1073/pnas.0306708101
- Primary Citation of Related Structures:
1S6V - PubMed Abstract:
A specific covalently cross-linked complex between redox partners yeast cytochrome c peroxidase (CCP) and cytochrome c (cyt. c) has been made by engineering cysteines into CCP and cyt. c that form an intermolecular disulfide bond in high yield. The crystal structure of the cross-linked complex has been solved to 1.88-A resolution and closely resembles the structure of the noncovalent complex [Pellitier, H. & Kraut, J. (1992) Science 258, 1748-1755]. The higher resolution of the covalent complex has enabled the location of ordered water molecules at the peroxidase-cytochrome c interface that serve to bridge between the two proteins by hydrogen bonding. As in the noncovalent complex, direct electrostatic interactions between protein groups appear not to be critical in complex formation. UV-visible spectroscopic and stopped-flow studies indicate that CCP in the covalent complex reacts normally with H(2)O(2) to give compound I. Stopped-flow kinetic studies also show that intramolecular electron transfer between the cross-linked ferrocytochrome c and the Trp-191 cation radical site in CCP compound I occurs fast and is nearly complete within the dead time ( approximately 2 ms) of the instrument. These results indicate that the structure of the covalent complex closely mimics the physiological electron transfer complex. In addition, single-turnover and steady-state experiments reveal that CCP compound I in the covalent complex oxidizes exogenously added ferrocytochrome c at a slow rate (t(1/2) approximately 2 min), indicating that CCP does not have a second independent site for physiologically relevant electron transfer.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, University of California, Irvine, CA 92697-3900, USA.