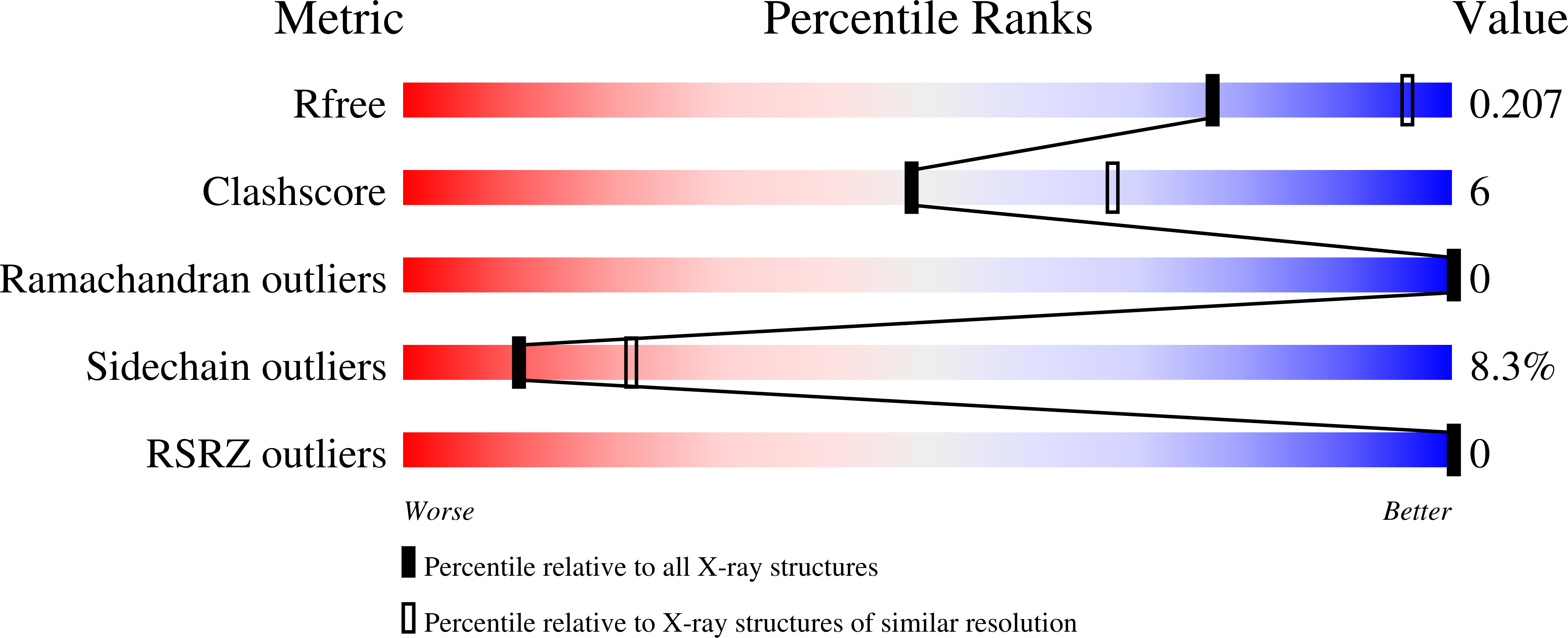
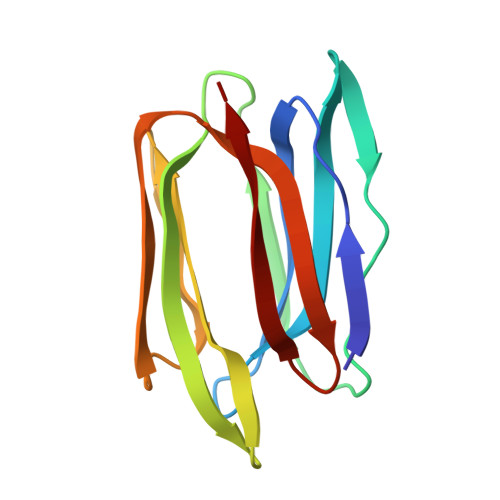
Two orthorhombic crystal structures of a galactose-specific lectin from Artocarpus hirsuta in complex with methyl-alpha-D-galactose.
Rao, K.N., Suresh, C.G., Katre, U.V., Gaikwad, S.M., Khan, M.I.(2004) Acta Crystallogr D Biol Crystallogr 60: 1404-1412
- PubMed: 15272163
- DOI: https://doi.org/10.1107/S090744490401354X
- Primary Citation of Related Structures:
1TOQ, 1TP8 - PubMed Abstract:
Based on their carbohydrate specificity, the jacalin family of lectins can be divided into two groups: galactose-specific and mannose-specific. The former are cytoplasmic proteins, whereas the latter are localized in the storage vacuoles of cells. It has been proposed that the post-translational modification in some of the lectins that splits their polypeptide chains into two may be crucial for galactose specificity. The mannose-specific members of the family are single-chain proteins that lack the above modification. Although the galactose-specific and the mannose-specific jacalin-type lectins differ in their sequences, they share a common fold: the beta-prism I fold, which is characteristic of Moraceae plant lectins. Here, two crystal structures of a jacalin-related lectin from Artocarpus hirsuta, which is specific for galactose, in complex with methyl-alpha-D-galactose are reported. The lectin crystallized in two orthorhombic forms and one hexagonal form under similar conditions. The crystals had an unusually high solvent content. The structure was solved using the molecular-replacement method using the jacalin structure as a search model. The two orthorhombic forms were refined using data to 2.5 and 3.0 A resolution, respectively. The structures of the A. hirsuta lectin and jacalin are identical. In orthorhombic form I the crystal packing provides three different micro-environments for sugar binding in the same crystal. The observed difference in the specificity for oligosaccharides between the A. hirsuta lectin and jacalin could only be explained based on differences in the molecular associations in the packing and variation of the C-terminal length of the beta-chain. The observed insecticidal activity of A. hirsuta lectin may arise from its similar fold to domain II of the unrelated delta-endotoxin from Bacillus thuringiensis.
Organizational Affiliation:
Division of Biochemical Sciences, National Chemical Laboratory, Pune-8, India.