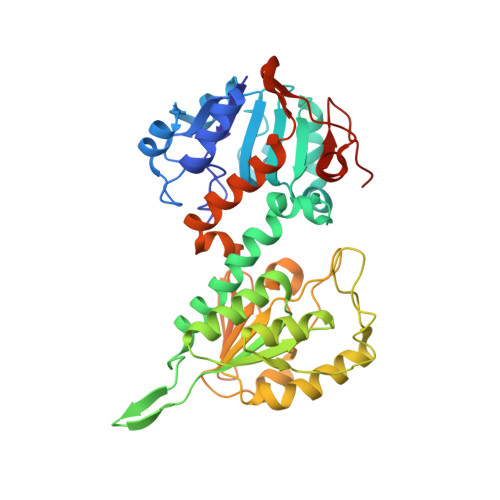
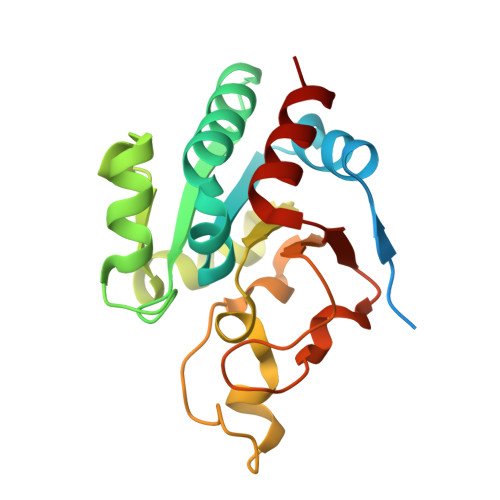
Active-site conformational changes associated with hydride transfer in proton-translocating transhydrogenase.
Mather, O.C., Singh, A., van Boxel, G.I., White, S.A., Jackson, J.B.(2004) Biochemistry 43: 10952-10964
- PubMed: 15323555
- DOI: https://doi.org/10.1021/bi0497594
- Primary Citation of Related Structures:
1U28, 1U2D, 1U2G, 1U31 - PubMed Abstract:
Transhydrogenase couples the redox (hydride-transfer) reaction between NAD(H) and NADP(H) to proton translocation across a membrane. The redox reaction is catalyzed at the interface between two components (dI and dIII) which protrude from the membrane. A complex formed from recombinant dI and dIII (the dI(2)dIII(1) complex) from Rhodospirillum rubrum transhydrogenase catalyzes fast single-turnover hydride transfer between bound nucleotides. In this report we describe three new crystal structures of the dI(2)dIII(1) complex in different nucleotide-bound forms. The structures reveal an asymmetry in nucleotide binding that complements results from solution studies and supports the notion that intact transhydrogenase functions by an alternating site mechanism. In one structure, the redox site is occupied by NADH (on dI) and NADPH (on dIII). The dihydronicotinamide rings take up positions which may approximate to the ground state for hydride transfer: the redox-active C4(N) atoms are separated by only 3.6 A, and the perceived reaction stereochemistry matches that observed experimentally. The NADH conformation is different in the two dI polypeptides of this form of the dI(2)dIII(1) complex. Comparisons between a number of X-ray structures show that a conformational change in the NADH is driven by relative movement of the two domains which comprise dI. It is suggested that an equivalent conformational change in the intact enzyme is important in gating the hydride-transfer reaction. The observed nucleotide conformational change in the dI(2)dIII(1) complex is accompanied by rearrangements in the orientation of local amino acid side chains which may be responsible for sealing the site from the solvent and polarizing hydride transfer.
Organizational Affiliation:
School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK.