Crystal Structure of Unsaturated Glucuronyl Hydrolase, Responsible for the Degradation of Glycosaminoglycan, from Bacillus sp. GL1 at 1.8 A Resolution
Itoh, T., Akao, S., Hashimoto, W., Mikami, B., Murata, K.(2004) J Biol Chem 279: 31804-31812
- PubMed: 15148314
- DOI: https://doi.org/10.1074/jbc.M403288200
- Primary Citation of Related Structures:
1VD5 - PubMed Abstract:
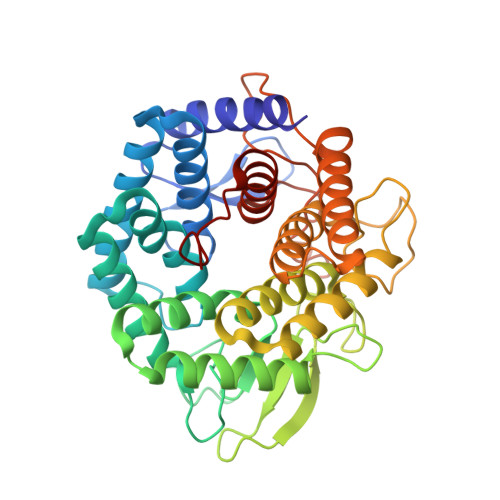
Unsaturated glucuronyl hydrolase (UGL) is a novel glycosaminoglycan hydrolase that releases unsaturated d-glucuronic acid from oligosaccharides produced by polysaccharide lyases. The x-ray crystallographic structure of UGL from Bacillus sp. GL1 was first determined by multiple isomorphous replacement (mir) and refined at 1.8 A resolution with a final R-factor of 16.8% for 25 to 1.8 A resolution data. The refined UGL structure consists of 377 amino acid residues and 478 water molecules, four glycine molecules, two dithiothreitol (DTT) molecules, and one 2-methyl-2,4-pentanediol (MPD) molecule. UGL includes an alpha(6)/alpha(6)-barrel, whose structure is found in the six-hairpin enzyme superfamily of an alpha/alpha-toroidal fold. One side of the UGL alpha(6)/alpha(6)-barrel structure consists of long loops containing three short beta-sheets and contributes to the formation of a deep pocket. One glycine molecule and two DTT molecules surrounded by highly conserved amino acid residues in UGLs were found in the pocket, suggesting that catalytic and substrate-binding sites are located in this pocket. The overall UGL structure, with the exception of some loops, very much resembled that of the Bacillus subtilis hypothetical protein Yter, whose function is unknown and which exhibits little amino acid sequence identity with UGL. In the active pocket, residues possibly involved in substrate recognition and catalysis by UGL are conserved in UGLs and Yter. The most likely candidate catalytic residues for glycosyl hydrolysis are Asp(88) and Asp(149). This was supported by site-directed mutagenesis studies in Asp(88) and Asp(149).
Organizational Affiliation:
Division of Agronomy and Horticultural Science, Graduate School of Agriculture, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan.