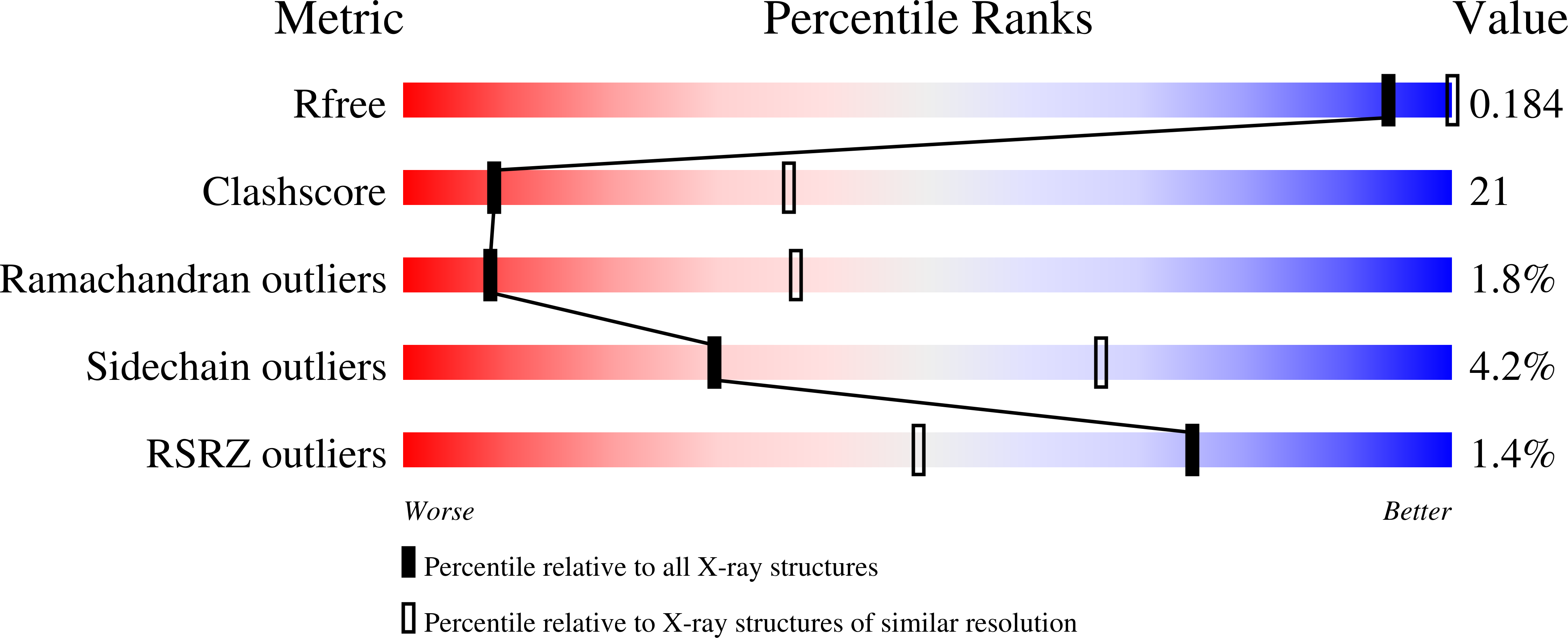
Structural insights into drug processing by human carboxylesterase 1: tamoxifen, mevastatin, and inhibition by benzil.
Fleming, C.D., Bencharit, S., Edwards, C.C., Hyatt, J.L., Tsurkan, L., Bai, F., Fraga, C., Morton, C.L., Howard-Williams, E.L., Potter, P.M., Redinbo, M.R.(2005) J Mol Biol 352: 165-177
- PubMed: 16081098
- DOI: https://doi.org/10.1016/j.jmb.2005.07.016
- Primary Citation of Related Structures:
1YA4, 1YA8, 1YAH, 1YAJ - PubMed Abstract:
Human carboxylesterase 1 (hCE1) exhibits broad substrate specificity and is involved in xenobiotic processing and endobiotic metabolism. We present and analyze crystal structures of hCE1 in complexes with the cholesterol-lowering drug mevastatin, the breast cancer drug tamoxifen, the fatty acyl ethyl ester (FAEE) analogue ethyl acetate, and the novel hCE1 inhibitor benzil. We find that mevastatin does not appear to be a substrate for hCE1, and instead acts as a partially non-competitive inhibitor of the enzyme. Similarly, we show that tamoxifen is a low micromolar, partially non-competitive inhibitor of hCE1. Further, we describe the structural basis for the inhibition of hCE1 by the nanomolar-affinity dione benzil, which acts by forming both covalent and non-covalent complexes with the enzyme. Our results provide detailed insights into the catalytic and non-catalytic processing of small molecules by hCE1, and suggest that the efficacy of clinical drugs may be modulated by targeted hCE1 inhibitors.
Organizational Affiliation:
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.