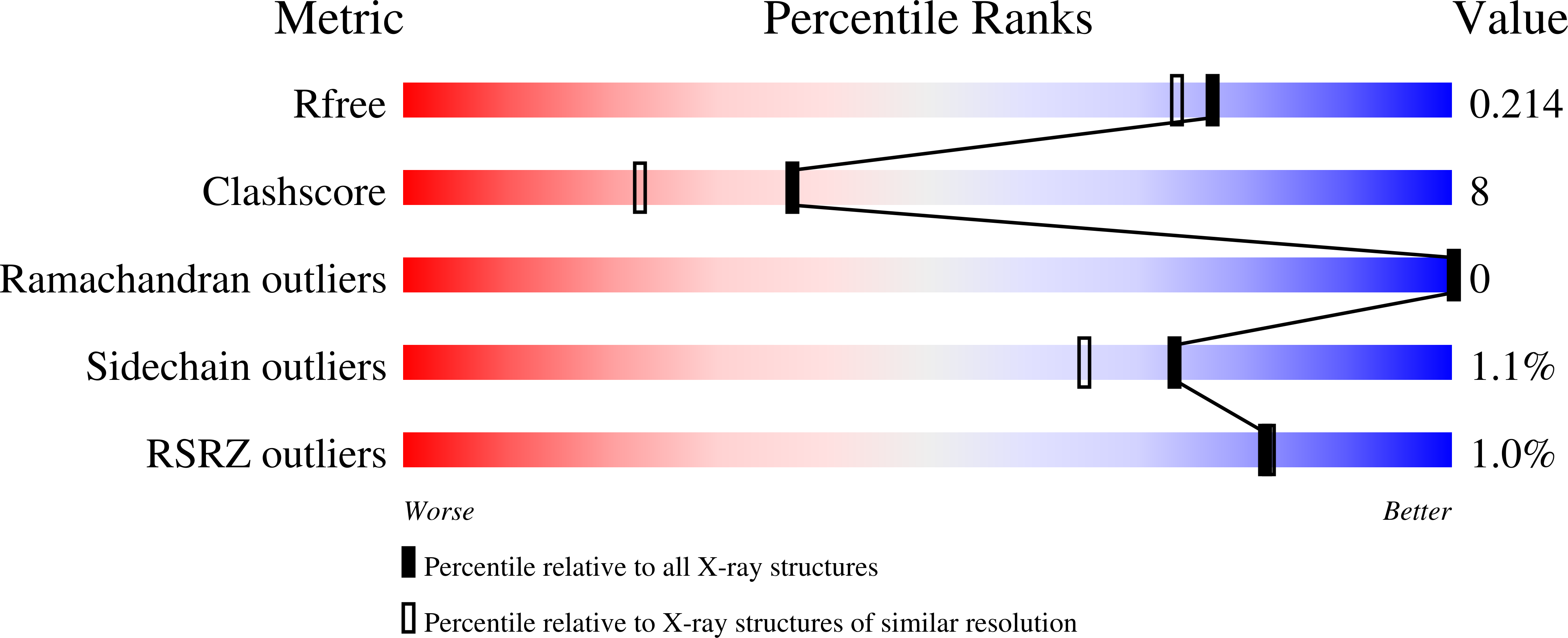
Seeing the process of histidine phosphorylation in human bisphosphoglycerate mutase.
Wang, Y., Liu, L., Wei, Z., Cheng, Z., Lin, Y., Gong, W.(2006) J Biol Chem 281: 39642-39648
- PubMed: 17052986
- DOI: https://doi.org/10.1074/jbc.M606421200
- Primary Citation of Related Structures:
2A9J, 2F90, 2H4X, 2H4Z, 2HHJ - PubMed Abstract:
Bisphosphoglycerate mutase is an erythrocyte-specific enzyme catalyzing a series of intermolecular phosphoryl group transfer reactions. Its main function is to synthesize 2,3-bisphosphoglycerate, the allosteric effector of hemoglobin. In this paper, we directly observed real-time motion of the enzyme active site and the substrate during phosphoryl transfer. A series of high resolution crystal structures of human bisphosphoglycerate mutase co-crystallized with 2,3-bisphosphoglycerate, representing different time points in the phosphoryl transfer reaction, were solved. These structures not only clarify the argument concerning the substrate binding mode for this enzyme family but also depict the entire process of the key histidine phosphorylation as a "slow movie". It was observed that the enzyme conformation continuously changed during the different states of the reaction. These results provide direct evidence for an "in line" phosphoryl transfer mechanism, and the roles of some key residues in the phosphoryl transfer process are identified.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.