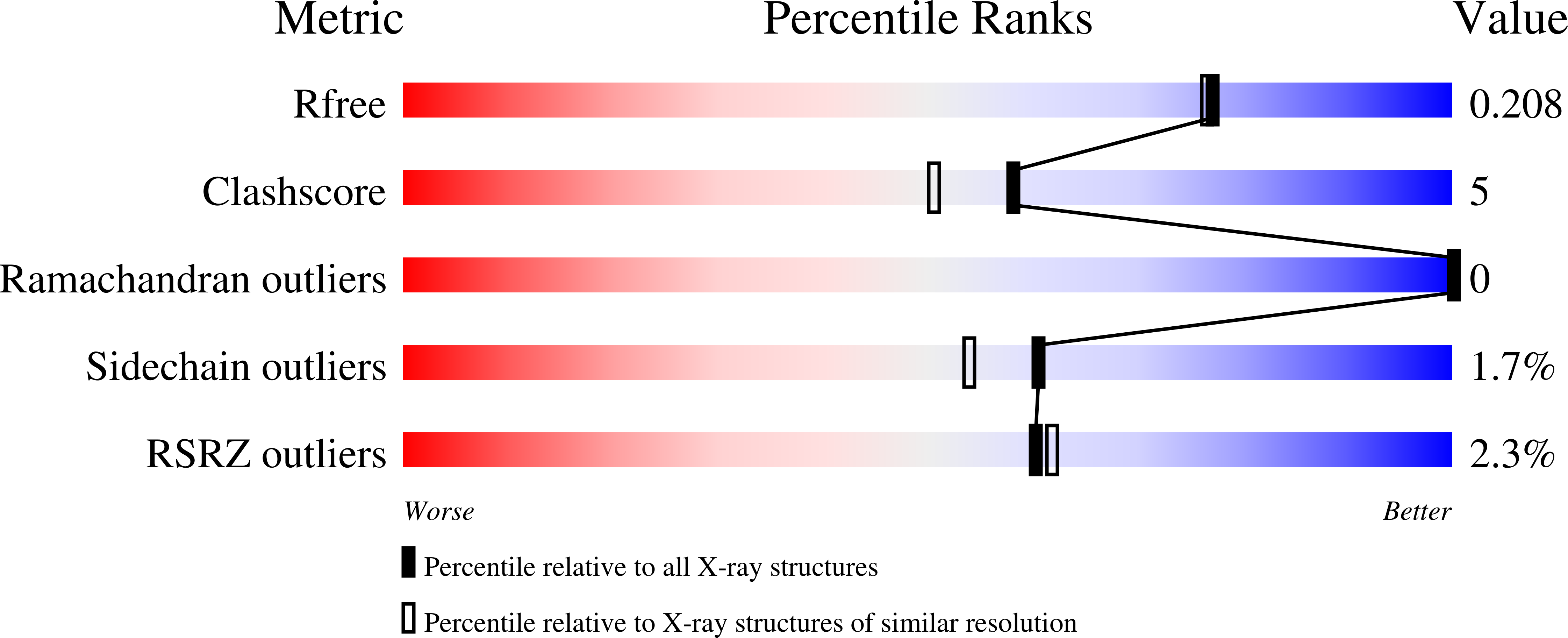
Potent and selective inhibitors of Helicobacter pylori glutamate racemase (MurI): pyridodiazepine amines.
Geng, B., Basarab, G., Comita-Prevoir, J., Gowravaram, M., Hill, P., Kiely, A., Loch, J., MacPherson, L., Morningstar, M., Mullen, G., Osimboni, E., Satz, A., Eyermann, C., Lundqvist, T.(2009) Bioorg Med Chem Lett 19: 930-936
- PubMed: 19097892
- DOI: https://doi.org/10.1016/j.bmcl.2008.11.113
- Primary Citation of Related Structures:
2W4I - PubMed Abstract:
An SAR study of an HTS screening hit generated a series of pyridodiazepine amines as potent inhibitors of Helicobacter pylori glutamate racemase (MurI) showing highly selective anti-H. pylori activity, marked improved solubility, and reduced plasma protein binding. X-ray co-crystal E-I structures were obtained. These uncompetitive inhibitors bind at the MurI dimer interface.
Organizational Affiliation:
AstraZeneca R&D Boston, Infection Discovery, 35 Gatehouse Drive, Waltham, MA 02451, USA. [email protected]