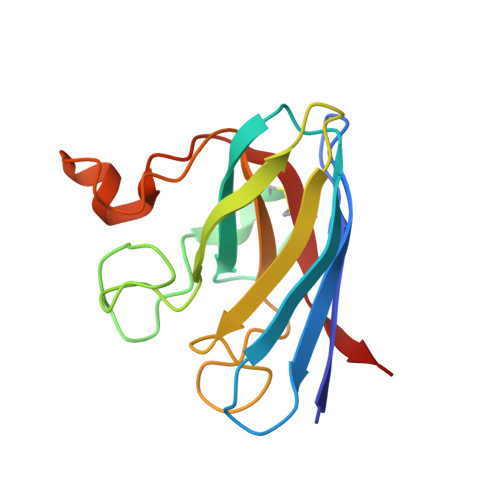
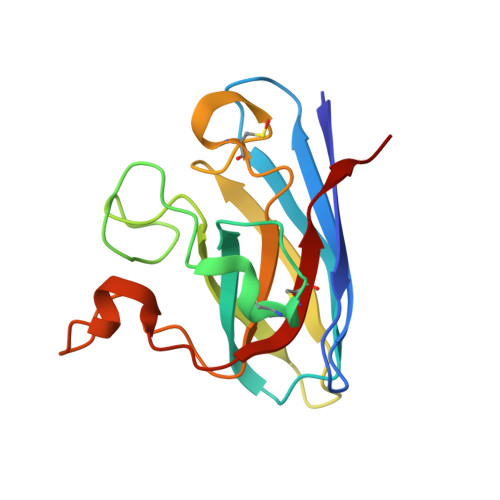
Structural and Biophysical Properties of Metal-Free Pathogenic Sod1 Mutants A4V and G93A.
Galaleldeen, A., Strange, R.W., Whitson, L.J., Antonyuk, S.V., Narayana, N., Taylor, A.B., Schuermann, J.P., Holloway, S.P., Hasnain, S.S., Hart, P.J.(2009) Arch Biochem Biophys 492: 40
- PubMed: 19800308
- DOI: https://doi.org/10.1016/j.abb.2009.09.020
- Primary Citation of Related Structures:
2WKO, 3GZO, 3GZP, 3GZQ - PubMed Abstract:
Amyotrophic lateral sclerosis (ALS) is a fatal, progressive neurodegenerative disease characterized by the destruction of motor neurons in the spinal cord and brain. A subset of ALS cases are linked to dominant mutations in copper-zinc superoxide dismutase (SOD1). The pathogenic SOD1 variants A4V and G93A have been the foci of multiple studies aimed at understanding the molecular basis for SOD1-linked ALS. The A4V variant is responsible for the majority of familial ALS cases in North America, causing rapidly progressing paralysis once symptoms begin and the G93A SOD1 variant is overexpressed in often studied murine models of the disease. Here we report the three-dimensional structures of metal-free A4V and of metal-bound and metal-free G93A SOD1. In the metal-free structures, the metal-binding loop elements are observed to be severely disordered, suggesting that these variants may share mechanisms of aggregation proposed previously for other pathogenic SOD1 proteins.
Organizational Affiliation:
Department of Biochemistry and the X-ray Crystallography Core Laboratory, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900, USA.