Crystal Structures of the Multidrug Binding Repressor Corynebacteriumglutamicum CgmR in Complex with Inducers and with an Operator
Itou, H., Watanabe, N., Yao, M., Shirakihara, Y., Tanaka, I.(2010) J Mol Biol 403: 174-184
- PubMed: 20691702
- DOI: https://doi.org/10.1016/j.jmb.2010.07.042
- Primary Citation of Related Structures:
2YVE, 2YVH, 2ZOZ - PubMed Abstract:
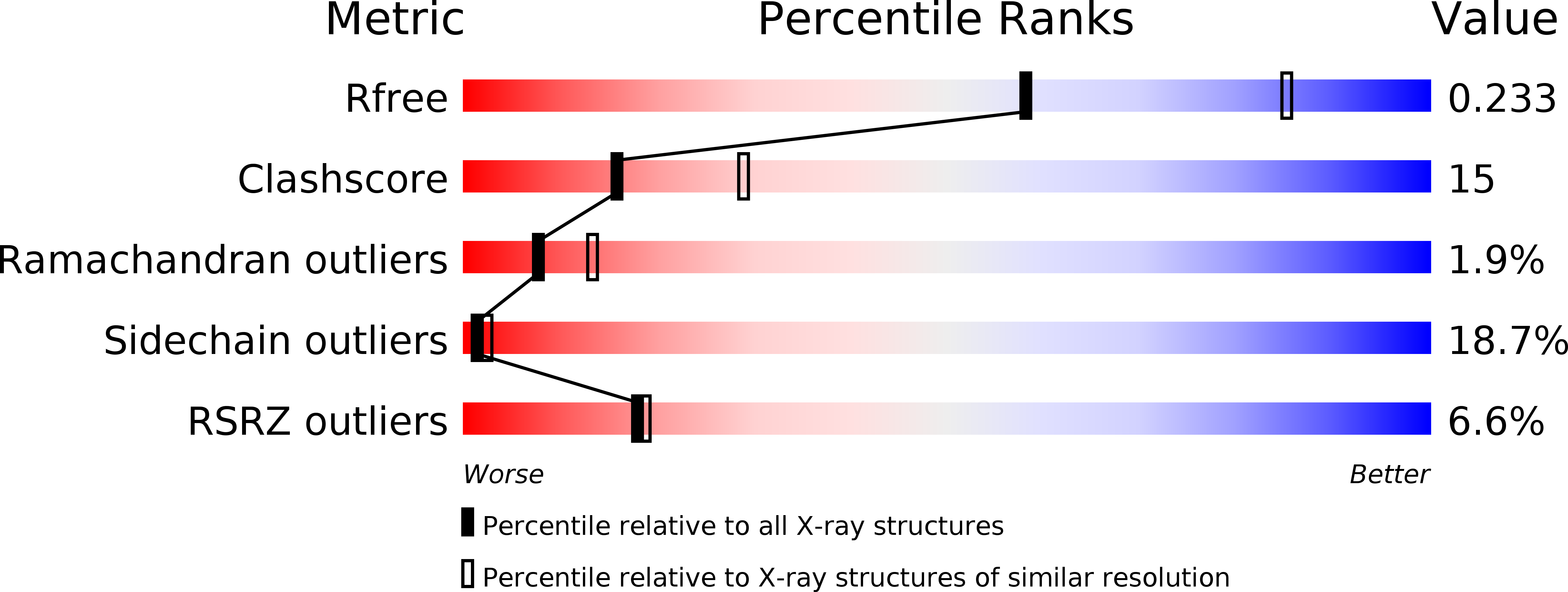
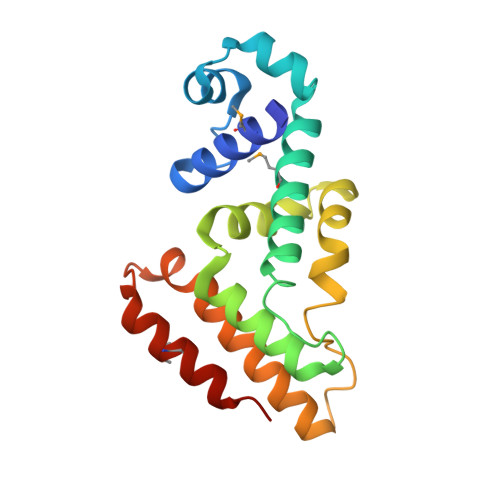
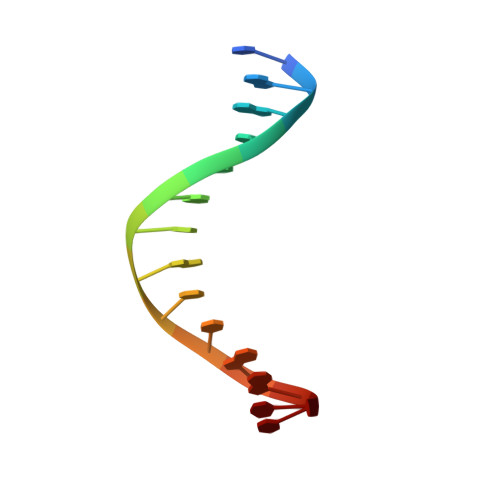
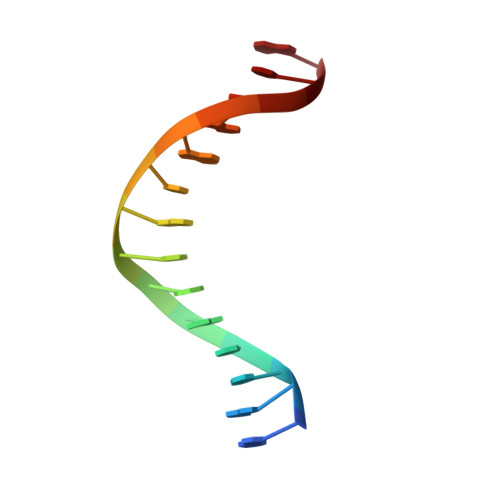
CgmR (CGL2612) from Corynebacterium glutamicum is a multidrug-resistance-related transcription factor belonging to the TetR family, which is a protein family of widespread bacterial transcription factors typically involved in environmental response. Here, we report the crystal structures of CgmR homodimeric repressor in complex with two distinct inducers (1.95 and 1.4 Å resolution) and with an operator (2.5 Å resolution). The CgmR-operator complex showed that two CgmR dimers bound to the operator, and each half-site of the palindromic operator was asymmetrically recognized by two DNA-binding domains from different dimers on the opposite sides of the DNA. The inducer complexes demonstrated that both bound inducers act as a wedge to alter the operator-binding conformation of the repressor by steric inhibition. As steric hindrance is used, various drugs should act as inducers if they have sufficient volume for the conformation change and if their bindings sufficiently reduce free energy. The comparative structural study of CgmR free protein, in complex with operator, and with inducers, implies the other mechanism that might contribute to multidrug response of the repressor.
Organizational Affiliation:
Structural Biology Center, National Institute of Genetics, Yata 1111, Mishima, Shizuoka 411-8540, Japan. [email protected]