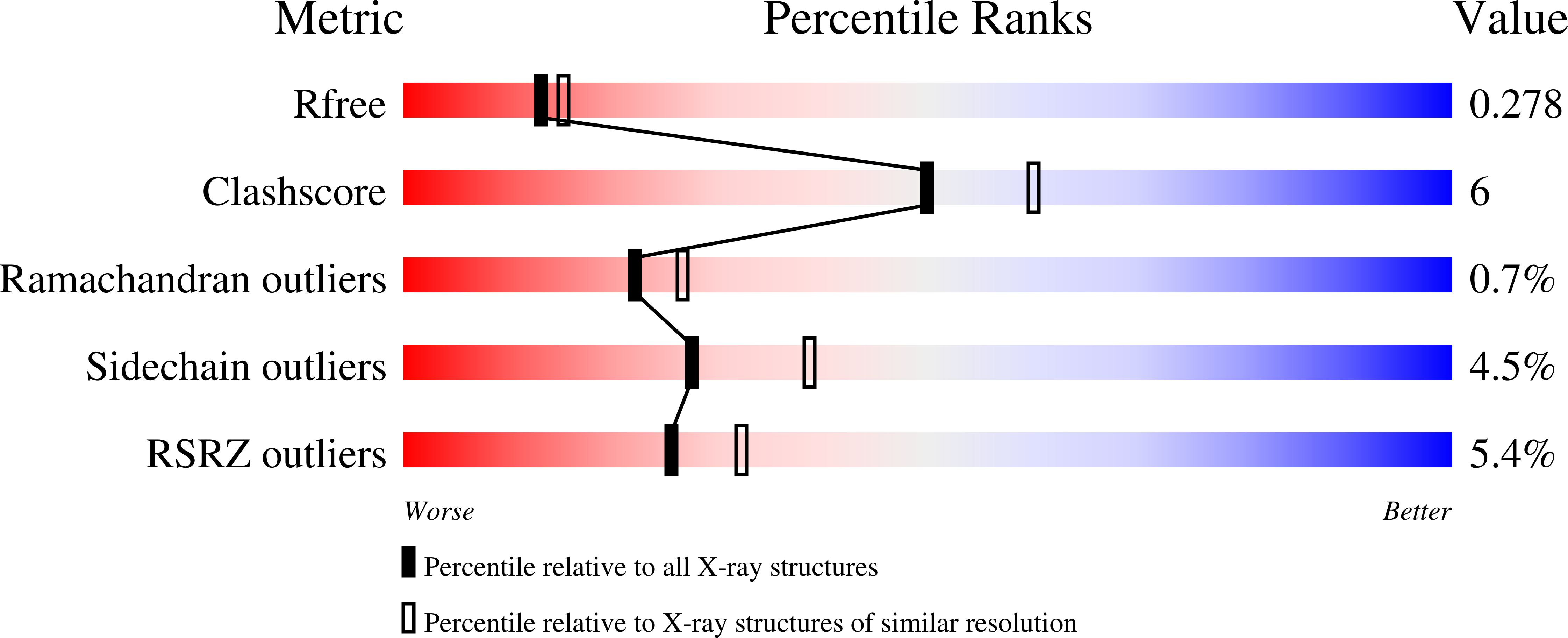
Protein conformational gating of enzymatic activity in xanthine oxidoreductase
Ishikita, H., Eger, B.T., Okamoto, K., Nishino, T., Pai, E.F.(2012) J Am Chem Soc 134: 999-1009
- PubMed: 22145797
- DOI: https://doi.org/10.1021/ja207173p
- Primary Citation of Related Structures:
3AX7, 3AX9, 3UNA, 3UNC, 3UNI - PubMed Abstract:
In mammals, xanthine oxidoreductase can exist as xanthine dehydrogenase (XDH) and xanthine oxidase (XO). The two enzymes possess common redox active cofactors, which form an electron transfer (ET) pathway terminated by a flavin cofactor. In spite of identical protein primary structures, the redox potential difference between XDH and XO for the flavin semiquinone/hydroquinone pair (E(sq/hq)) is ~170 mV, a striking difference. The former greatly prefers NAD(+) as ultimate substrate for ET from the iron-sulfur cluster FeS-II via flavin while the latter only accepts dioxygen. In XDH (without NAD(+)), however, the redox potential of the electron donor FeS-II is 180 mV higher than that for the acceptor flavin, yielding an energetically uphill ET. On the basis of new 1.65, 2.3, 1.9, and 2.2 Å resolution crystal structures for XDH, XO, the NAD(+)- and NADH-complexed XDH, E(sq/hq) were calculated to better understand how the enzyme activates an ET from FeS-II to flavin. The majority of the E(sq/hq) difference between XDH and XO originates from a conformational change in the loop at positions 423-433 near the flavin binding site, causing the differences in stability of the semiquinone state. There was no large conformational change observed in response to NAD(+) binding at XDH. Instead, the positive charge of the NAD(+) ring, deprotonation of Asp429, and capping of the bulk surface of the flavin by the NAD(+) molecule all contribute to altering E(sq/hq) upon NAD(+) binding to XDH.
Organizational Affiliation:
Career-Path Promotion Unit for Young Life Scientists, Kyoto University, 202 Building E, Graduate School of Medicine, Kyoto 606-8501, Japan. [email protected]