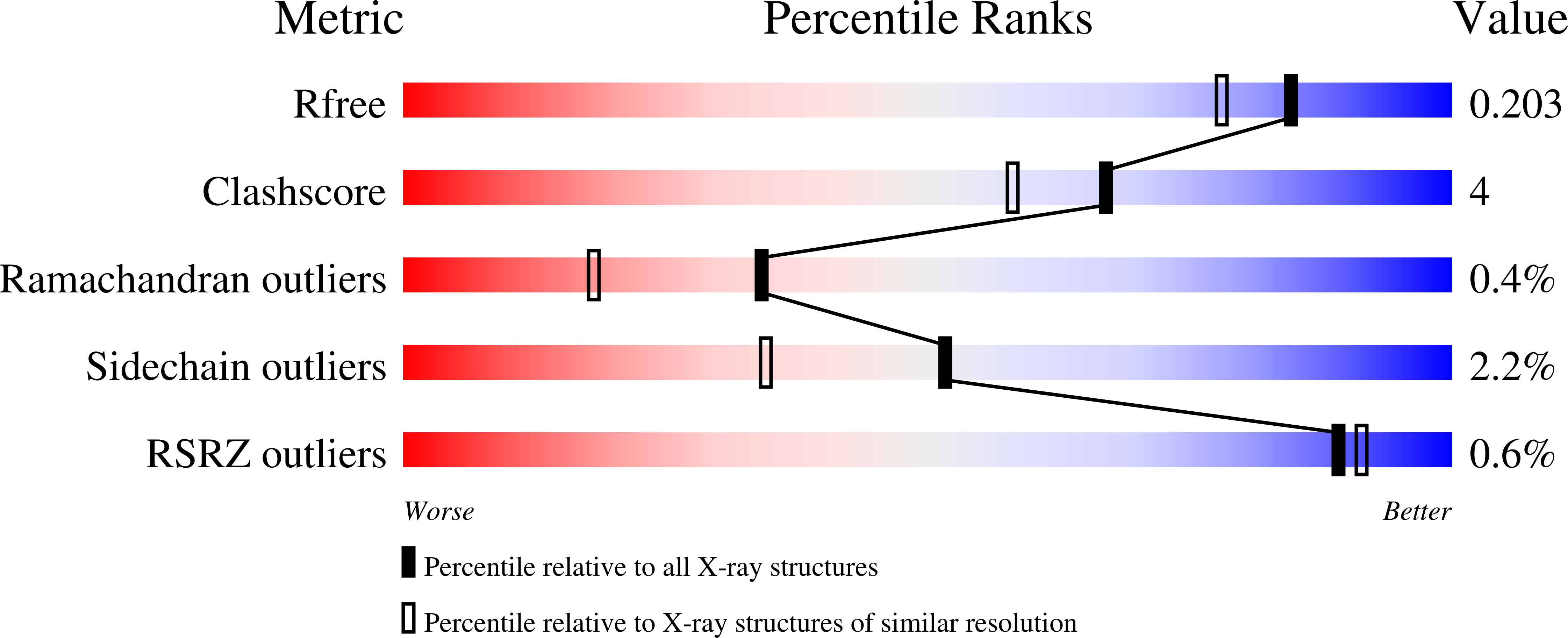
Molecular docking and ligand specificity in fragment-based inhibitor discovery
Chen, Y., Shoichet, B.K.(2009) Nat Chem Biol 5: 358-364
- PubMed: 19305397
- DOI: https://doi.org/10.1038/nchembio.155
- Primary Citation of Related Structures:
3G2Y, 3G2Z, 3G30, 3G31, 3G32, 3G34, 3G35 - PubMed Abstract:
Fragment screens have successfully identified new scaffolds in drug discovery, often with relatively high hit rates (5%) using small screening libraries (1,000-10,000 compounds). This raises two questions: would other noteworthy chemotypes be found were one to screen all commercially available fragments (>300,000), and does the success rate imply low specificity of fragments? We used molecular docking to screen large libraries of fragments against CTX-M beta-lactamase. We identified ten millimolar-range inhibitors from the 69 compounds tested. The docking poses corresponded closely to the crystallographic structures subsequently determined. Notably, these initial low-affinity hits showed little specificity between CTX-M and an unrelated beta-lactamase, AmpC, which is unusual among beta-lactamase inhibitors. This is consistent with the idea that the high hit rates among fragments correlate to a low initial specificity. As the inhibitors were progressed, both specificity and affinity rose together, yielding to our knowledge the first micromolar-range noncovalent inhibitors against a class A beta-lactamase.
Organizational Affiliation:
Department of Pharmaceutical Chemistry, University of California, San Francisco, California, USA.