Glyphosate resistance by engineering the flavoenzyme glycine oxidase.
Pedotti, M., Rosini, E., Molla, G., Moschetti, T., Savino, C., Vallone, B., Pollegioni, L.(2009) J Biol Chem 284: 36415-36423
- PubMed: 19864430
- DOI: https://doi.org/10.1074/jbc.M109.051631
- Primary Citation of Related Structures:
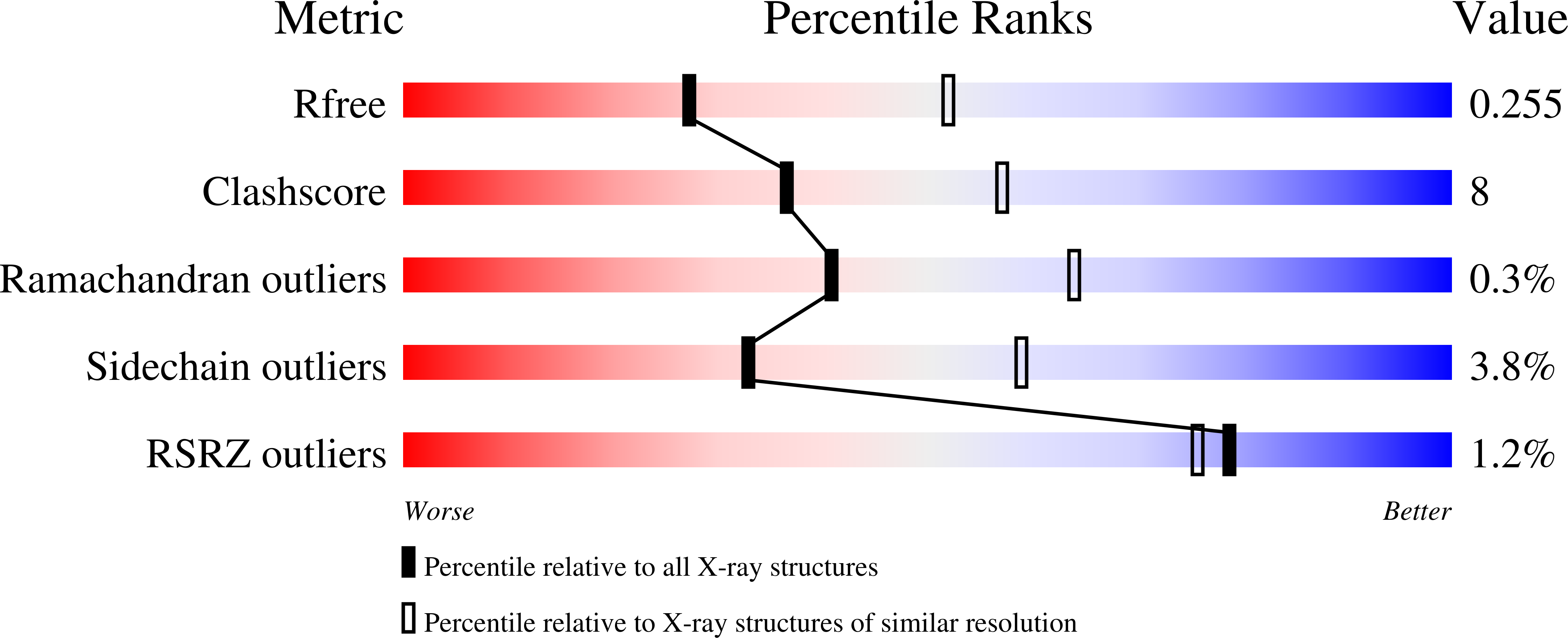
3IF9 - PubMed Abstract:
Glycine oxidase from Bacillus subtilis is a homotetrameric flavoprotein of great potential biotechnological use because it catalyzes the oxidative deamination of various amines and d-isomer of amino acids to yield the corresponding alpha-keto acids, ammonia/amine, and hydrogen peroxide. Glyphosate (N-phosphonomethylglycine), a broad spectrum herbicide, is an interesting synthetic amino acid: this compound inhibits 5-enolpyruvylshikimate-3-phosphate synthase in the shikimate pathway, which is essential for the biosynthesis of aromatic amino acids in plants and certain bacteria. In recent years, transgenic crops resistant to glyphosate were mainly generated by overproducing the plant enzyme or by introducing a 5-enolpyruvylshikimate-3-phosphate synthase insensitive to this herbicide. In this work, we propose that the enzymatic oxidation of glyphosate could be an effective alternative to this important biotechnological process. To reach this goal, we used a rational design approach (together with site saturation mutagenesis) to generate a glycine oxidase variant more active on glyphosate than on the physiological substrate glycine. The glycine oxidase containing three point mutations (G51S/A54R/H244A) reaches an up to a 210-fold increase in catalytic efficiency and a 15,000-fold increase in the specificity constant (the k(cat)/K(m) ratio between glyphosate and glycine) as compared with wild-type glycine oxidase. The inspection of its three-dimensional structure shows that the alpha2-alpha3 loop (comprising residues 50-60 and containing two of the mutated residues) assumes a novel conformation and that the newly introduced residue Arg(54) could be the key residue in stabilizing glyphosate binding and destabilizing glycine positioning in the binding site, thus increasing efficiency on the herbicide.
Organizational Affiliation:
Dipartimento di Biotecnologie e Scienze Molecolari and the Centro Interuniversitario di Ricerca in Biotecnologie Proteiche "The Protein Factory," Politecnico di Milano, Università degli Studi dell'Insubria, 21100 Varese, Italy.