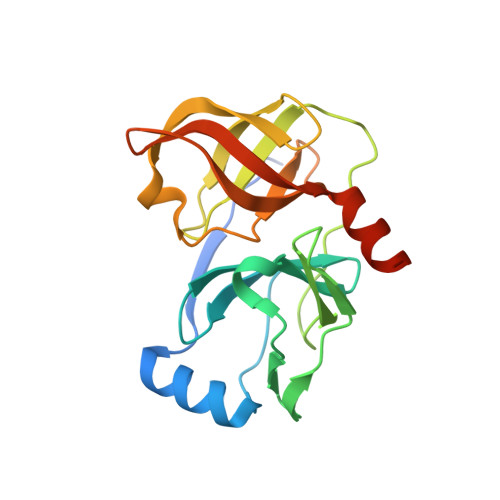
The introduction of P4 substituted 1-methylcyclohexyl groups into Boceprevir: a change in direction in the search for a second generation HCV NS3 protease inhibitor.
Bennett, F., Huang, Y., Hendrata, S., Lovey, R., Bogen, S.L., Pan, W., Guo, Z., Prongay, A., Chen, K.X., Arasappan, A., Venkatraman, S., Velazquez, F., Nair, L., Sannigrahi, M., Tong, X., Pichardo, J., Cheng, K.C., Girijavallabhan, V.M., Saksena, A.K., Njoroge, F.G.(2010) Bioorg Med Chem Lett 20: 2617-2621
- PubMed: 20303756
- DOI: https://doi.org/10.1016/j.bmcl.2010.02.063
- Primary Citation of Related Structures:
3LOX - PubMed Abstract:
In the search for a second generation HCV protease inhibitor, molecular modeling studies of the X-ray crystal structure of Boceprevir1 bound to the NS3 protein suggest that expansion into the S4 pocket could provide additional hydrophobic Van der Waals interactions. Effective replacement of the P4 tert-butyl with a cyclohexylmethyl ligand led to inhibitor 2 with improved enzyme and replicon activities. Subsequent modeling and SAR studies led to the pyridine 38 and sulfone analogues 52 and 53 with vastly improved PK parameters in monkeys, forming a new foundation for further exploration.
Organizational Affiliation:
Schering-Plough Research Institute, K-15-A-3545, Kenilworth, NJ 07033, USA. [email protected]