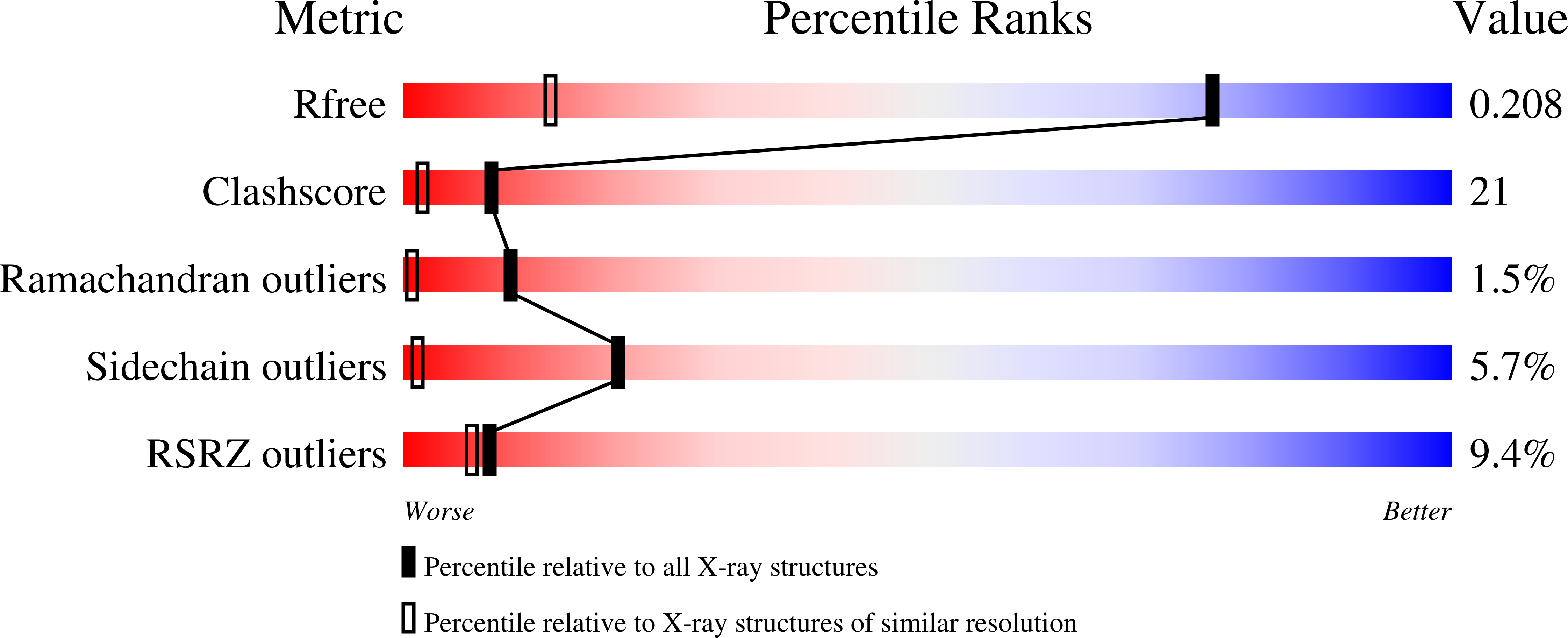
Cooperative binding of MgATP and MgADP in the trimeric P(II) protein GlnK2 from Archaeoglobus fulgidus.
Helfmann, S., Lu, W., Litz, C., Andrade, S.L.(2010) J Mol Biol 402: 165-177
- PubMed: 20643148
- DOI: https://doi.org/10.1016/j.jmb.2010.07.020
- Primary Citation of Related Structures:
3NCP, 3NCQ, 3NCR - PubMed Abstract:
P(II)-like proteins, such as GlnK, found in a wide variety of organisms from prokaryotes to plants constitute a family of cytoplasmic signaling proteins that play a central regulatory role in the assimilation of nitrogen for biosyntheses. They specifically bind and are modulated by effector molecules such as adenosine triphosphate, adenosine diphosphate and 2-oxoglutarate. Their highly conserved, trimeric structure suggests that cooperativity in effector binding might be the basis for the ability to integrate and respond to a wide range of concentrations, but to date no direct quantification of this cooperative behavior has been presented. The hyperthermophilic archaeon Archaeoglobus fulgidus contains three GlnK proteins, functionally associated with ammonium transport proteins (Amt). We have characterized GlnK2 and its interaction with effectors by high-resolution X-ray crystallography and isothermal titration calorimetry. Binding of adenosine nucleotides resulted in distinct, cooperative behavior for ATP and ADP. While 2-oxoglutarate has been shown to interact with other GlnK proteins, GlnK2 was completely insensitive to this key indicator of a low level of intracellular nitrogen. These findings point to different regulation and modulation patterns and add to our understanding of the flexibility and versatility of the GlnK family of signaling proteins.
Organizational Affiliation:
Institut für organische Chemieund Biochemie, Albert-Ludwigs-Universität Freiburg, Albertstr.21, 79104 Freiburg, Germany.