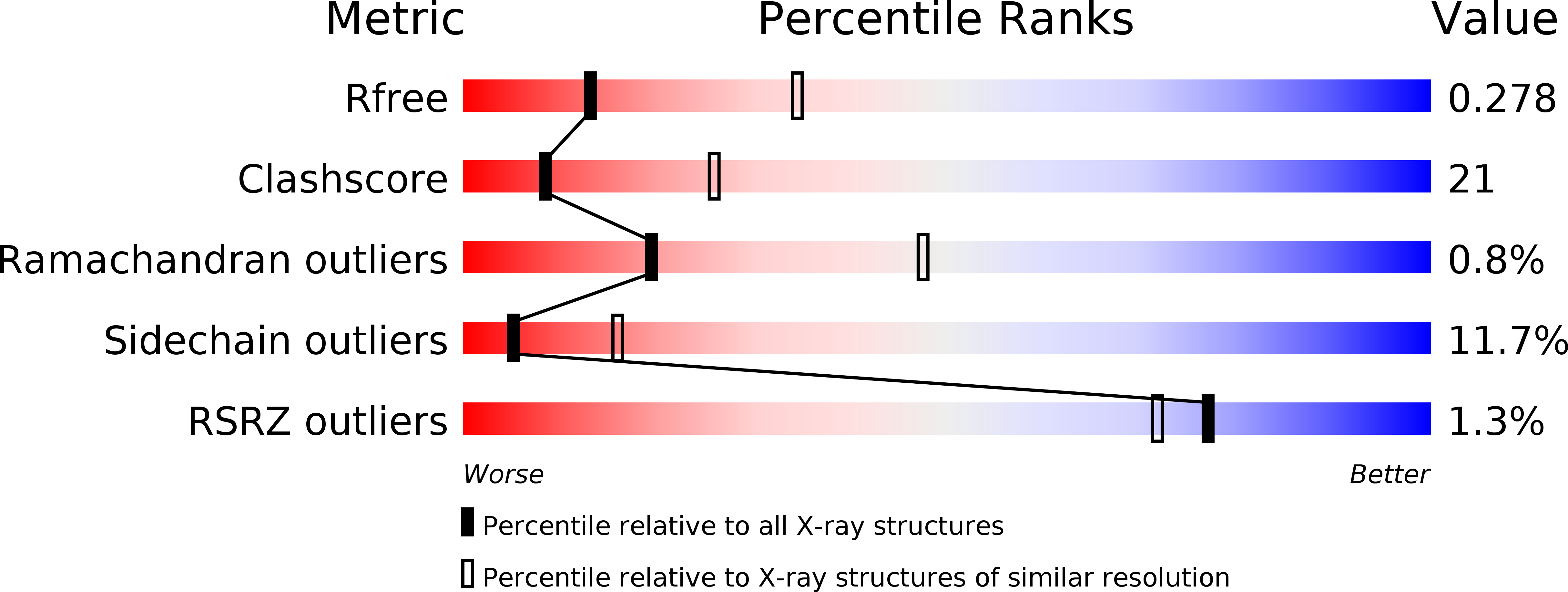
Crystal structure of Bacteroides thetaiotaomicron TetX2: a tetracycline degrading monooxygenase at 2.8 A resolution.
Walkiewicz, K., Davlieva, M., Wu, G., Shamoo, Y.(2011) Proteins 79: 2335-2340
- PubMed: 21590745
- DOI: https://doi.org/10.1002/prot.23052
- Primary Citation of Related Structures:
3P9U - PubMed Abstract:
We have determined the structure of Bacteroides thetaiotaomicron TetX2 at 2.8 Å resolution, and shown that it is a class A flavin dependent oxidoreductase. TetX2 has broad activity against a range of tetracyclines including one of the most recent tetracyclines, tigecycline (Tygacil ® ). Comparison of TetX2 with that of the weakly homologous Pseudomonas fluorescens para-hydroxybenzoate hydroxylase (PHBH) (21% identity) shows substantial differences among residues at the substrate binding site although FAD is positioned in a similar conformation between the two enzymes and is poised to carry out catalysis.
Organizational Affiliation:
Department of Biochemistry and Cell Biology, Rice University, 6100 Main St. MS-140, Houston, Texas, USA.