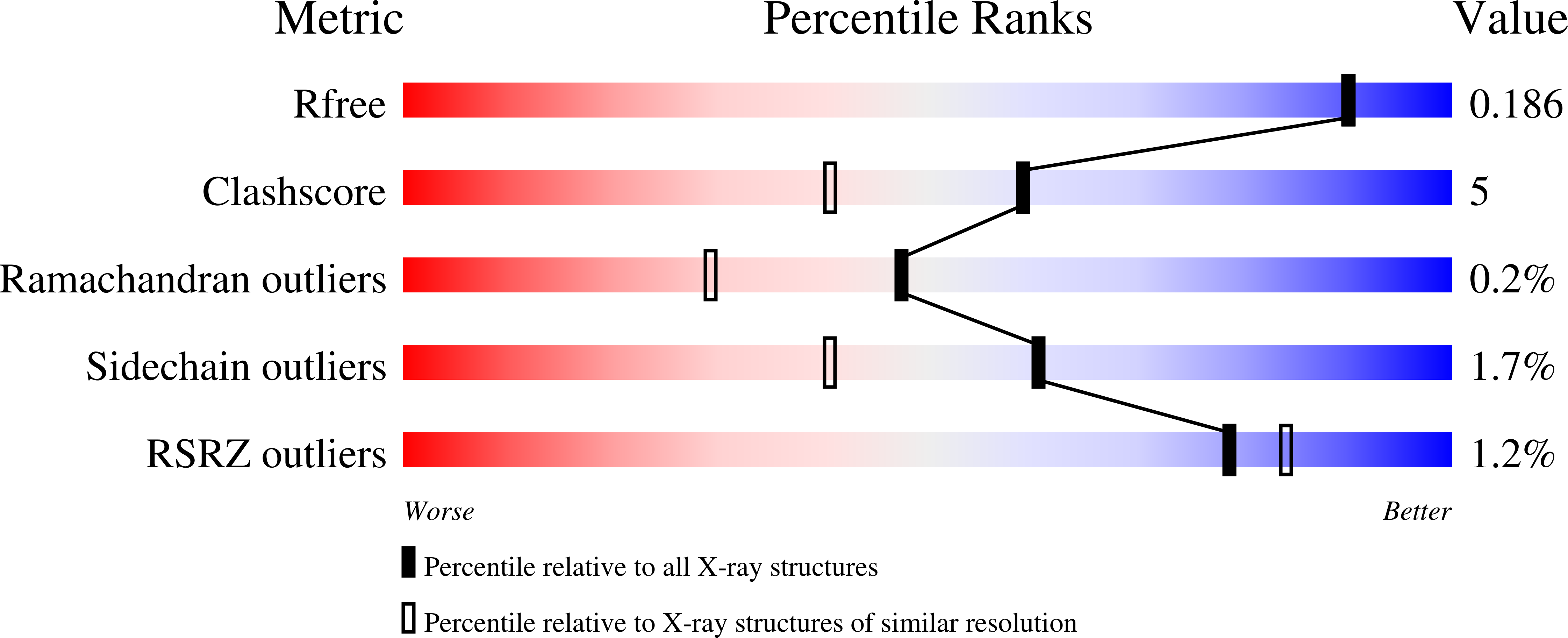
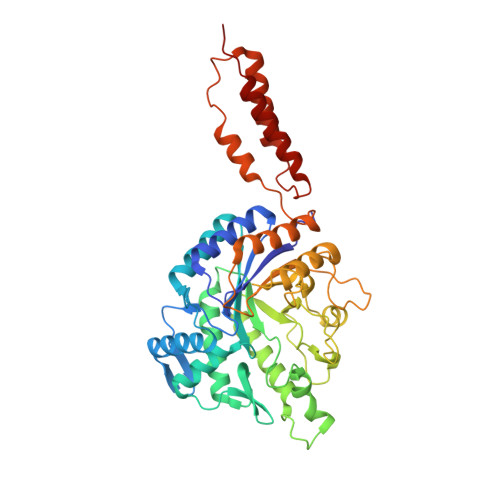
Inhibition of the Family 20 Glycoside Hydrolase Catalytic Modules in the Streptococcus Pneumoniae Exo-Beta-D-N-Acetylglucosaminidase, Strh.
Pluvinage, B., Stubbs, K.A., Vocadlo, D.J., Boraston, A.B.(2013) Org Biomol Chem 11: 7907
- PubMed: 24132305
- DOI: https://doi.org/10.1039/c3ob41579a
- Primary Citation of Related Structures:
4AZ5, 4AZ6, 4AZ7, 4AZB, 4AZC, 4AZG, 4AZH, 4AZI - PubMed Abstract:
Streptococcus pneumoniae produces a cell-surface attached β-N-acetylglucosaminidase called StrH that is used by this pathogen to process the termini of host complex N-linked glycans. N-Acetyl-D-glucosamine-thiazoline (NAG-Thiazoline, NGT) and O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino N-phenyl carbamate (PUGNAc) are inhibitors of the two family 20 glycoside hydrolase catalytic modules within StrH and these inhibitors have proven useful in modulating the activity of StrH in assays that model aspects of the host-bacterium interaction. Here we explore the molecular basis of StrH inhibition through structural, kinetic, thermodynamic and site-directed mutagenic analyses using the recombinantly produced independent catalytic modules of StrH (GH20A and GH20B) and the inhibitors NGT and PUGNAc. The results reveal a similar binding mode of the sugar moiety of these inhibitors at the -1 subsite in the active sites of GH20A and GH20B. The lower affinity of NGT as compared to PUGNAc for these catalytic modules can be attributed to the hydrophobic phenylcarbamate moiety of PUGNAc that is absent in NGT. This moiety also displayed variations in its interactions with the active sites of GH20A and GH20B that provide a rationale for the 400-fold difference observed in the Ki values of this compound for these two β-N-acetylglucosaminidase catalytic modules.
Organizational Affiliation:
Biochemistry & Microbiology, University of Victoria, PO Box 3055 STN CSC, Victoria, BC V8W 3P6, Canada. [email protected].