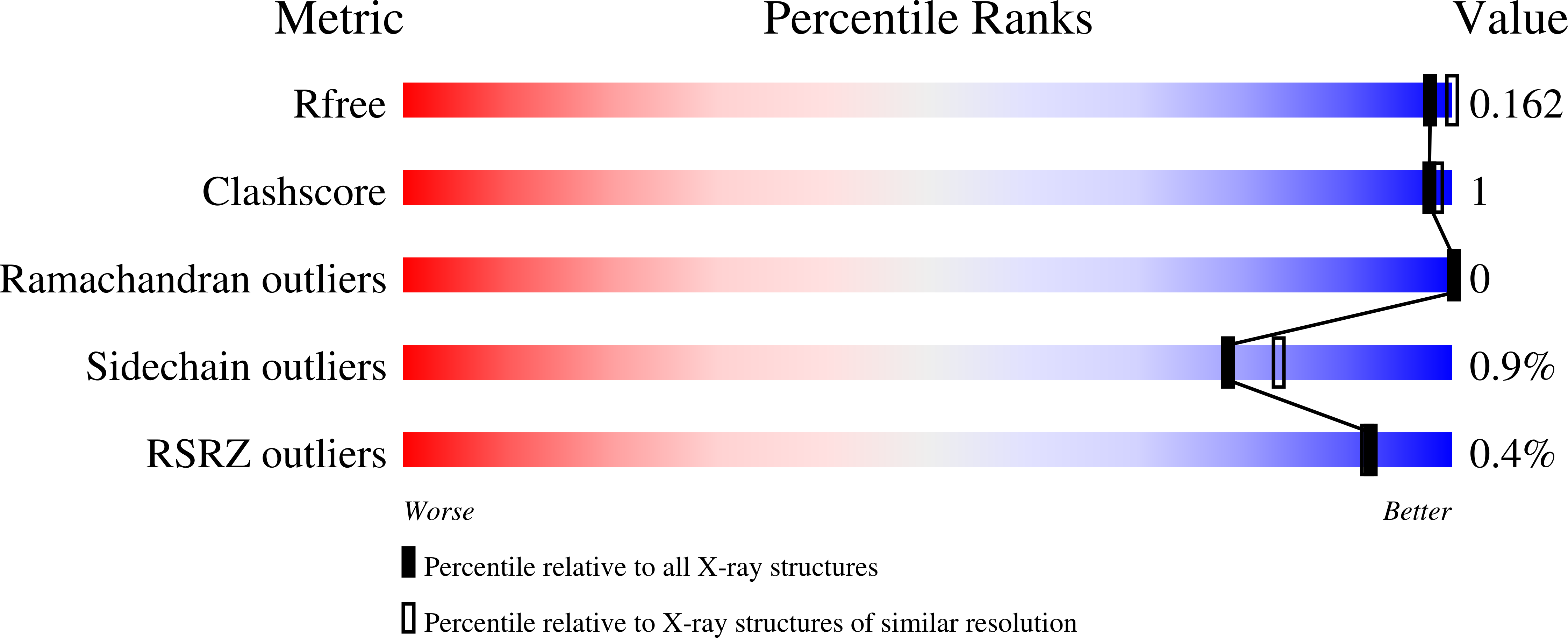
A Novel I221 L Substitution in Neuraminidase Confers High Level Resistance to Oseltamivir in Influenza B Viruses.
Escuret, V., Collins, P.J., Casalegno, J., Vachieri, S.G., Cattle, N., Ferraris, O., Sabatier, M., Frobert, E., Caro, V., Skehel, J.J., Gamblin, S., Valla, F., Valette, M., Ottmann, M., Mccauley, J.W., Daniels, R.S., Lina, B.(2014) J Infect Dis 210: 1260
- PubMed: 24795482
- DOI: https://doi.org/10.1093/infdis/jiu244
- Primary Citation of Related Structures:
4CPL, 4CPM, 4CPN, 4CPO, 4CPY, 4CPZ - PubMed Abstract:
Influenza B viruses with a novel I221L substitution in neuraminidase (NA) conferring high-level resistance to oseltamivir were isolated from an immunocompromised patient after prolonged oseltamivir treatment. Enzymatic characterization of the NAs (Km, Ki) and the in vitro fitness of viruses carrying wild-type or mutated (I221L) NA genes were evaluated. Proportions of wild-type and mutated NA genes were directly quantified in the patient samples. Structural characterizations by X-ray crystallography of a wild-type and I221L variant NA were performed. The Km and Ki revealed that the I221L variant NA had approximately 84 and 51 times lower affinity for oseltamivir carboxylate and zanamivir, respectively, compared with wild-type NA. Viruses with a wild-type or I221L variant NA had similar growth kinetics in Madin-Darby canine kidney (MDCK) cells, and 5 passages in MDCK cells revealed no reversion of the I221L substitution. The crystal structure of the I221L NA and oseltamivir complex showed that the leucine side chain protrudes into the hydrophobic pocket of the active site that accommodates the pentyloxy substituent of oseltamivir. Enzyme kinetic and NA structural analyses provide an explanation for the high level of resistance to oseltamivir while retaining good fitness of viruses carrying I221L variant NA.
Organizational Affiliation:
Laboratoire de Virologie et Centre National de Référence virus influenzae Laboratoire Virpath EA4610, Faculté de Médecine Lyon Est, Université Claude Bernard Lyon 1, Université de Lyon, and.