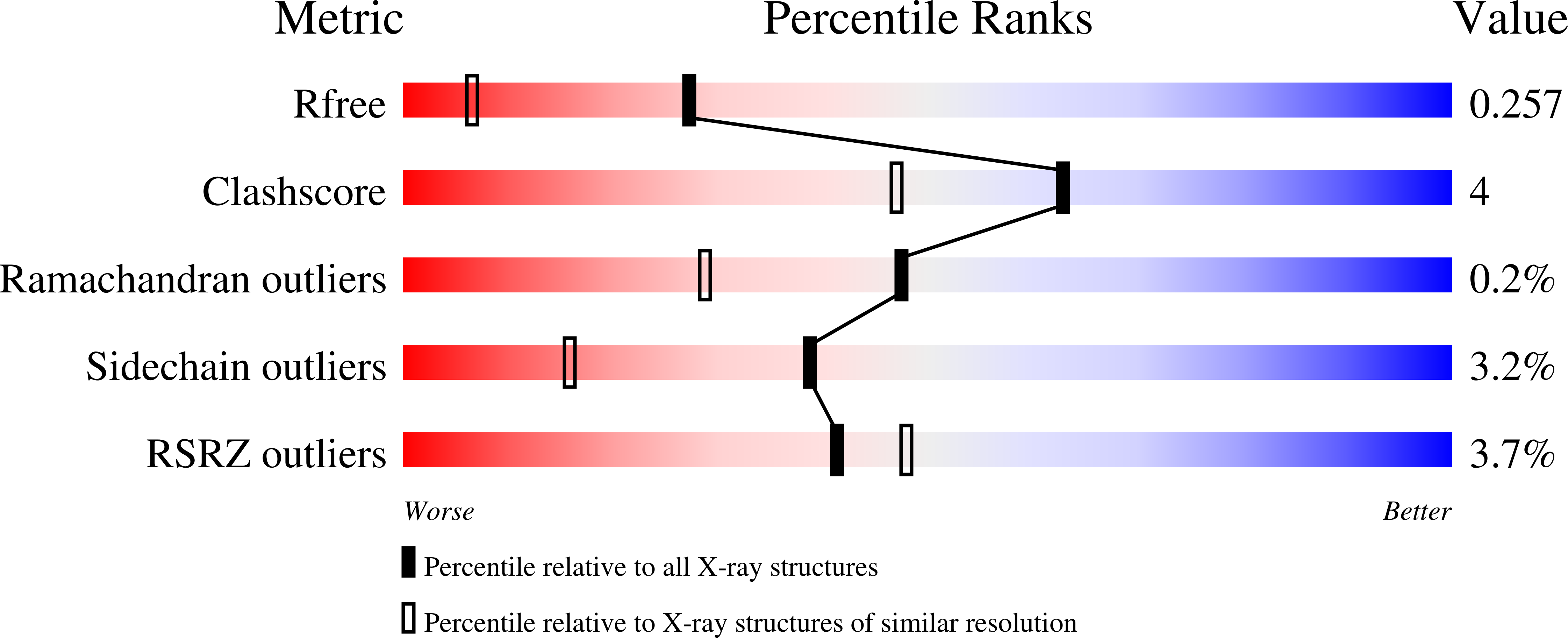
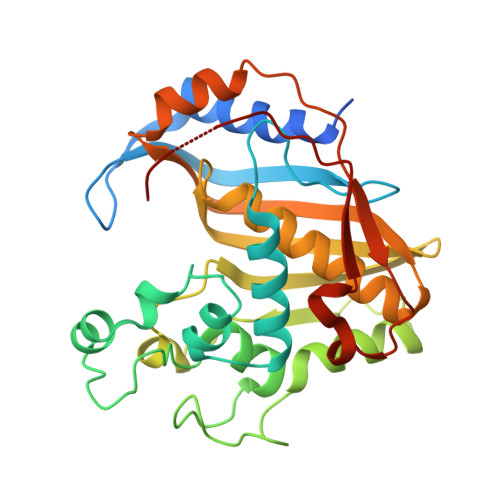
Crystal structures of complexes of mouse thymidylate synthase crystallized with N4-OH-dCMP alone or in the presence of N5,10-methylenetetrahydrofolate
Dowiercial, A., Jarmula, A., Rypniewski, W.R., Wilk, P., Kierdaszuk, B., Rode, W.(2013) Pteridines