Structure-based identification of ureas as novel nicotinamide phosphoribosyltransferase (nampt) inhibitors.
Zheng, X., Bauer, P., Baumeister, T., Buckmelter, A.J., Caligiuri, M., Clodfelter, K.H., Han, B., Ho, Y.C., Kley, N., Lin, J., Reynolds, D.J., Sharma, G., Smith, C.C., Wang, Z., Dragovich, P.S., Oh, A., Wang, W., Zak, M., Gunzner-Toste, J., Zhao, G., Yuen, P.W., Bair, K.W.(2013) J Med Chem 56: 4921-4937
- PubMed: 23617784
- DOI: https://doi.org/10.1021/jm400186h
- Primary Citation of Related Structures:
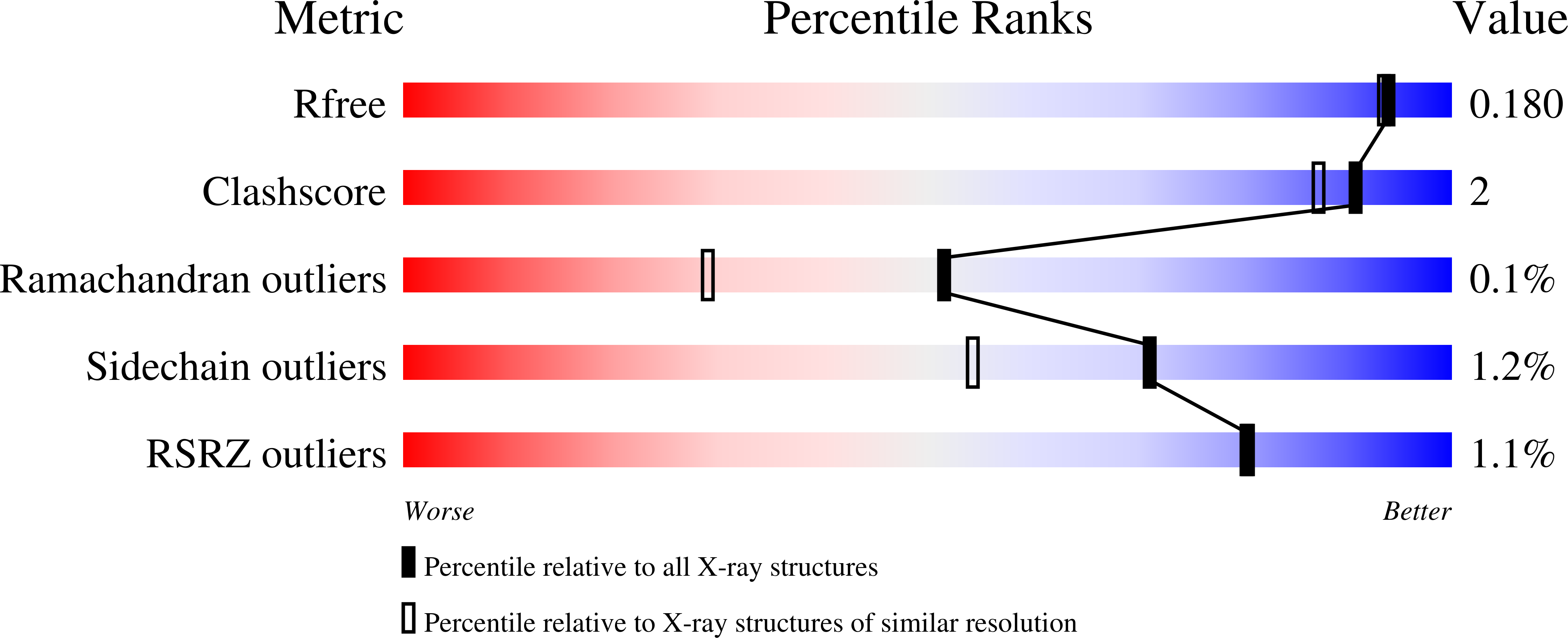
4JR5, 4KFN, 4KFO - PubMed Abstract:
Nicotinamide phosphoribosyltransferase (Nampt) is a promising anticancer target. Virtual screening identified a thiourea analogue, compound 5, as a novel highly potent Nampt inhibitor. Guided by the cocrystal structure of 5, SAR exploration revealed that the corresponding urea compound 7 exhibited similar potency with an improved solubility profile. These studies also indicated that a 3-pyridyl group was the preferred substituent at one inhibitor terminus and also identified a urea moiety as the optimal linker to the remainder of the inhibitor structure. Further SAR optimization of the other inhibitor terminus ultimately yielded compound 50 as a urea-containing Nampt inhibitor which exhibited excellent biochemical and cellular potency (enzyme IC50 = 0.007 μM; A2780 IC50 = 0.032 μM). Compound 50 also showed excellent in vivo antitumor efficacy when dosed orally in an A2780 ovarian tumor xenograft model (TGI of 97% was observed on day 17).
Organizational Affiliation:
Forma Therapeutics, Inc., 500 Arsenal Street, Watertown, Massachusetts 02472, USA. [email protected]