Effects of zinc on particulate methane monooxygenase activity and structure.
Sirajuddin, S., Barupala, D., Helling, S., Marcus, K., Stemmler, T.L., Rosenzweig, A.C.(2014) J Biol Chem 289: 21782-21794
- PubMed: 24942740
- DOI: https://doi.org/10.1074/jbc.M114.581363
- Primary Citation of Related Structures:
4PHZ, 4PI0, 4PI2 - PubMed Abstract:
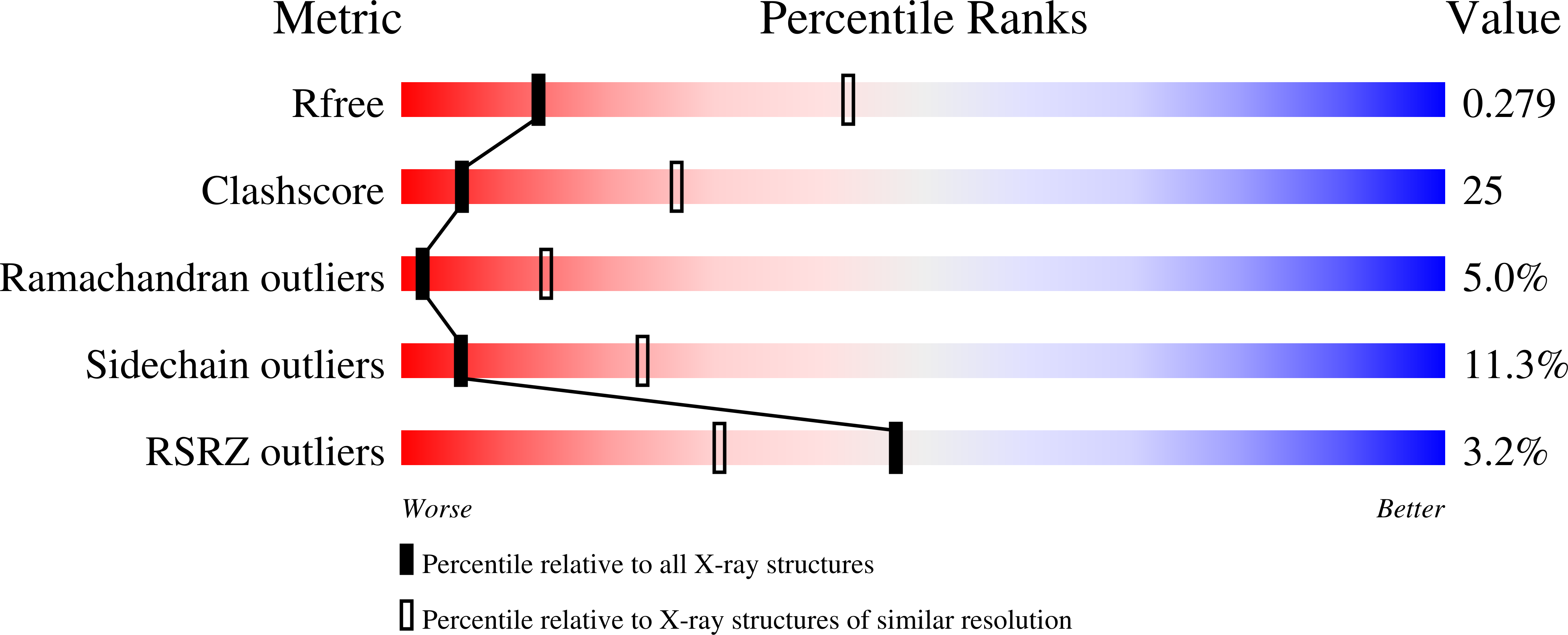
Particulate methane monooxygenase (pMMO) is a membrane-bound metalloenzyme that oxidizes methane to methanol in methanotrophic bacteria. Zinc is a known inhibitor of pMMO, but the details of zinc binding and the mechanism of inhibition are not understood. Metal binding and activity assays on membrane-bound pMMO from Methylococcus capsulatus (Bath) reveal that zinc inhibits pMMO at two sites that are distinct from the copper active site. The 2.6 Å resolution crystal structure of Methylocystis species strain Rockwell pMMO reveals two previously undetected bound lipids, and metal soaking experiments identify likely locations for the two zinc inhibition sites. The first is the crystallographic zinc site in the pmoC subunit, and zinc binding here leads to the ordering of 10 previously unobserved residues. A second zinc site is present on the cytoplasmic side of the pmoC subunit. Parallels between these results and zinc inhibition studies of several respiratory complexes suggest that zinc might inhibit proton transfer in pMMO.
Organizational Affiliation:
From the Departments of Molecular Biosciences and of Chemistry, Northwestern University, Evanston, Illinois 60208.