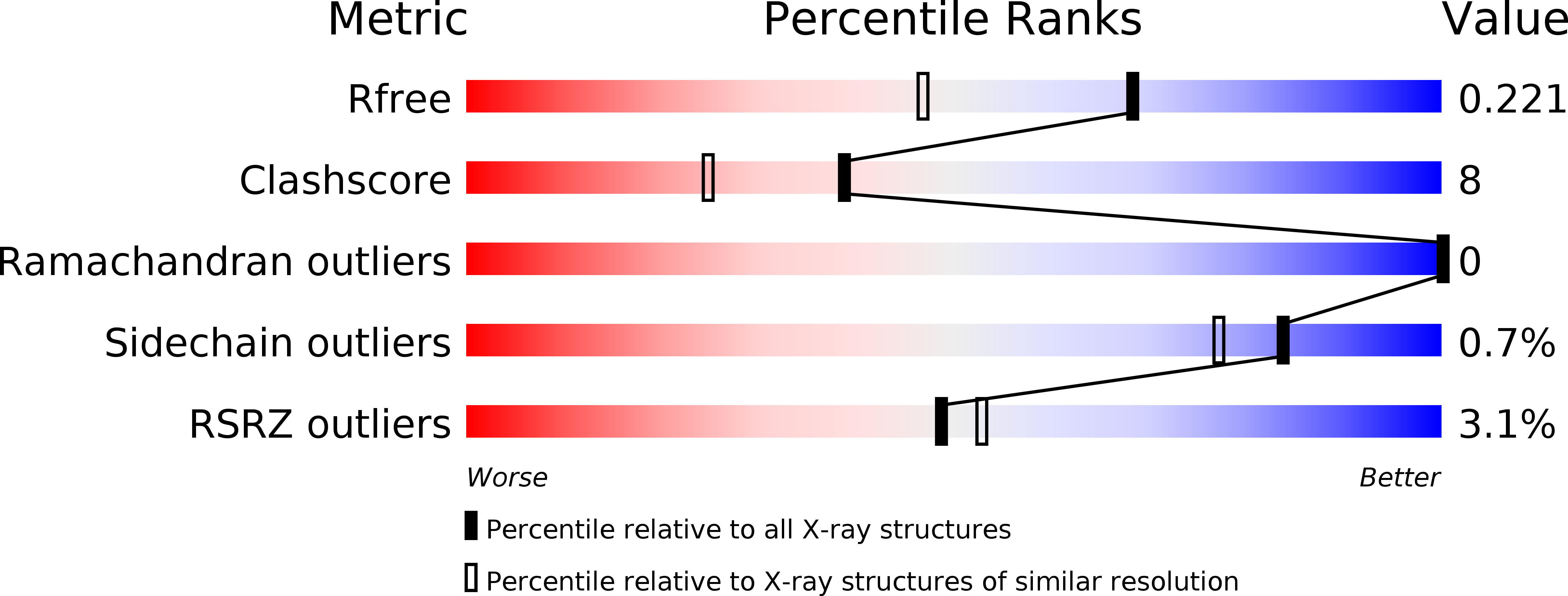
Structural Analysis of Asunaprevir Resistance in HCV NS3/4A Protease.
Soumana, D.I., Ali, A., Schiffer, C.A.(2014) ACS Chem Biol 9: 2485-2490
- PubMed: 25243902
- DOI: https://doi.org/10.1021/cb5006118
- Primary Citation of Related Structures:
4WF8, 4WH6, 4WH8 - PubMed Abstract:
Asunaprevir (ASV), an isoquinoline-based competitive inhibitor targeting the hepatitis C virus (HCV) NS3/4A protease, is very potent in vivo. However, the potency is significantly compromised by the drug resistance mutations R155K and D168A. In this study three crystal structures of ASV and an analogue were determined to analyze the structural basis of drug resistance susceptibility. These structures revealed that ASV makes extensive contacts with Arg155 outside the substrate envelope. Arg155 in turn is stabilized by Asp168, and thus when either residue is mutated, the enzyme's interaction with ASV's P2* isoquinoline is disrupted. Adding a P1-P3 macrocycle to ASV enhances the inhibitor's resistance barrier, likely due to poising the inhibitor to its bound conformation. Macrocyclic inhibitors with P2* extension moieties avoiding interaction with the protease S2 residues including Arg155 must be chosen for future design of more robust protease inhibitors.
Organizational Affiliation:
Department of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School , Worcester, Massachusetts 01655, United States.