Microcrystal delivery by pulsed liquid droplet for serial femtosecond crystallography.
Mafune, F., Miyajima, K., Tono, K., Takeda, Y., Kohno, J.Y., Miyauchi, N., Kobayashi, J., Joti, Y., Nango, E., Iwata, S., Yabashi, M.(2016) Acta Crystallogr D Struct Biol 72: 520-523
- PubMed: 27050131
- DOI: https://doi.org/10.1107/S2059798316001480
- Primary Citation of Related Structures:
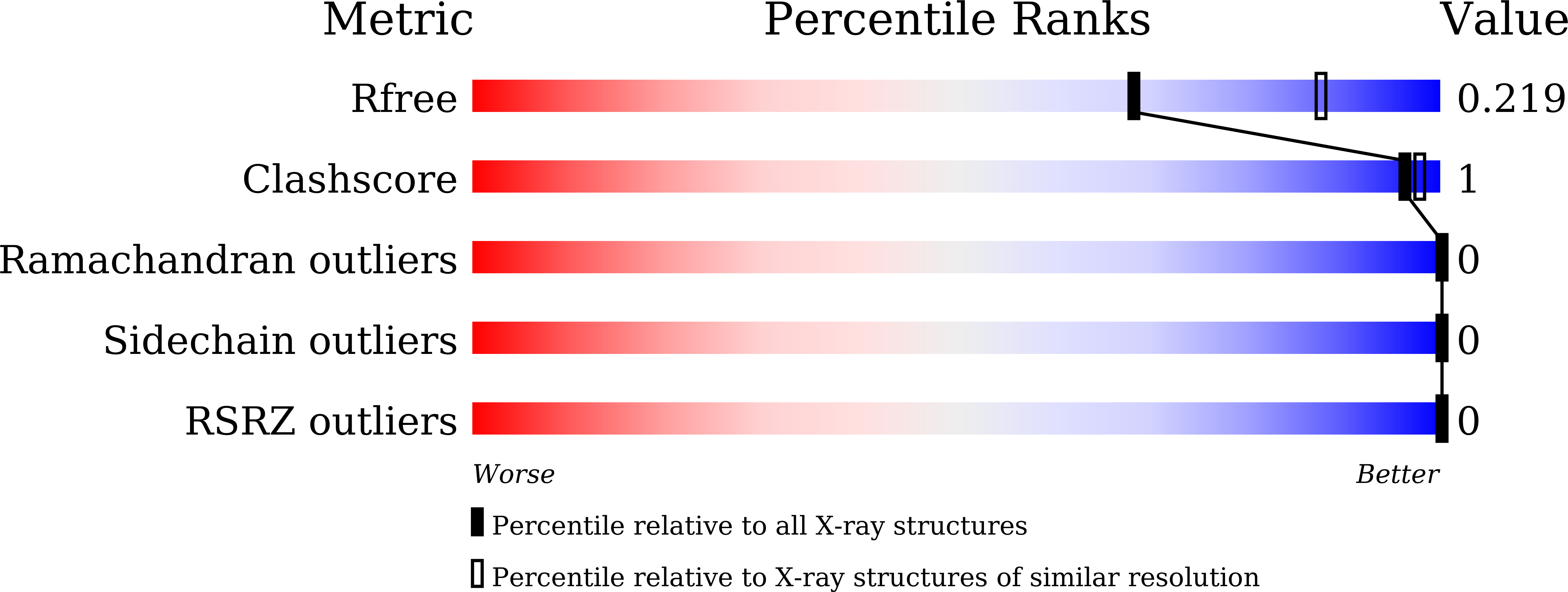
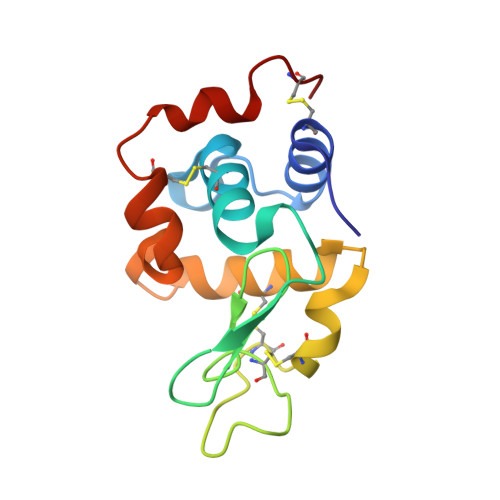
5DM9 - PubMed Abstract:
A liquid-droplet injector has been developed that delivers pristine microcrystals to an X-ray irradiation area for conducting serial femtosecond crystallography (SFX) with an X-ray free-electron laser (XFEL). By finely tuning the pulsed liquid droplets in time and space, a high hit rate of the XFEL pulses to microcrystals in the droplets was achieved for measurements using 5 µm tetragonal lysozyme crystals, which produced 4265 indexable diffraction images in about 30 min. The structure was determined at a resolution of 2.3 Å from <0.3 mg of protein. With further improvements such as reduction of the droplet size, liquid droplets have considerable potential as a crystal carrier for SFX with low sample consumption.
Organizational Affiliation:
Department of Basic Science, School of Arts and Sciences, The University of Tokyo, Komaba, Meguro, Tokyo 153-8902, Japan.