Correlation of structure, function and protein dynamics in GH7 cellobiohydrolases from Trichoderma atroviride, T. reesei and T. harzianum.
Borisova, A.S., Eneyskaya, E.V., Jana, S., Badino, S.F., Kari, J., Amore, A., Karlsson, M., Hansson, H., Sandgren, M., Himmel, M.E., Westh, P., Payne, C.M., Kulminskaya, A.A., Stahlberg, J.(2018) Biotechnol Biofuels 11: 5-5
- PubMed: 29344086
- DOI: https://doi.org/10.1186/s13068-017-1006-7
- Primary Citation of Related Structures:
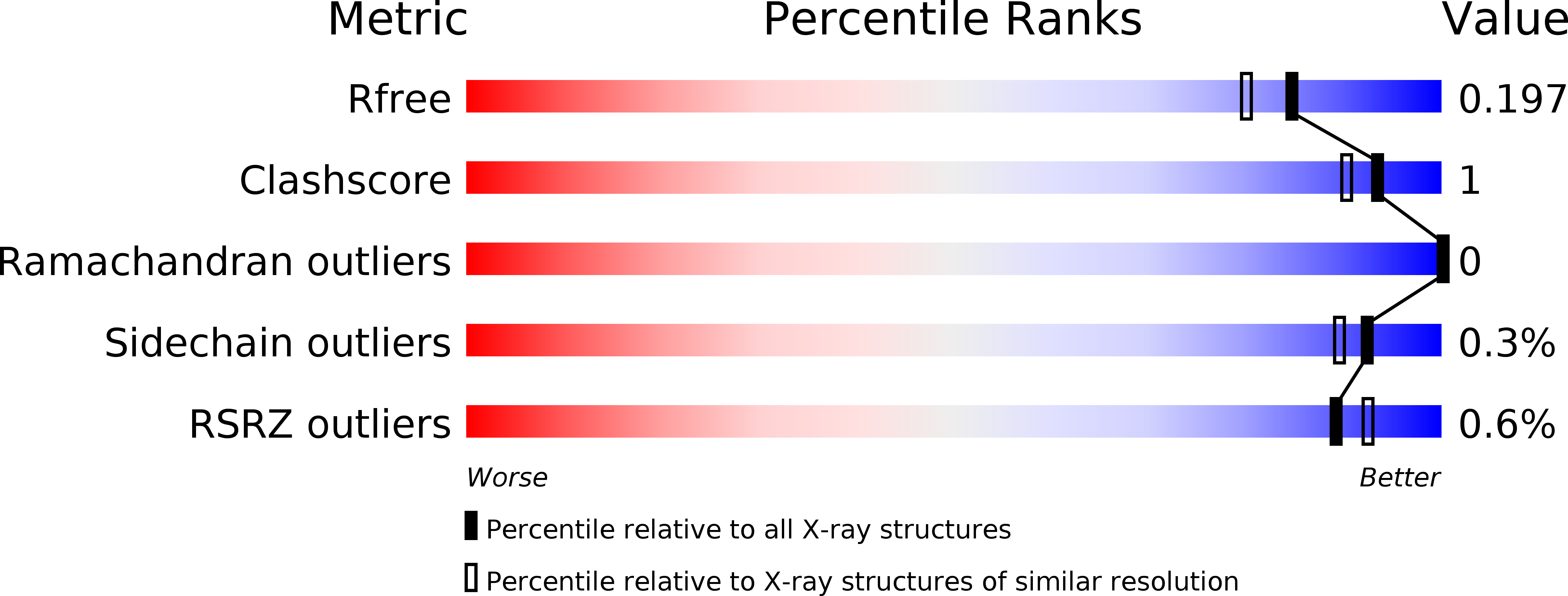
5O59, 5O5D - PubMed Abstract:
The ascomycete fungus Trichoderma reesei is the predominant source of enzymes for industrial conversion of lignocellulose. Its glycoside hydrolase family 7 cellobiohydrolase (GH7 CBH) Tre Cel7A constitutes nearly half of the enzyme cocktail by weight and is the major workhorse in the cellulose hydrolysis process. The orthologs from Trichoderma atroviride ( Tat Cel7A) and Trichoderma harzianum ( Tha Cel7A) show high sequence identity with Tre Cel7A, ~ 80%, and represent naturally evolved combinations of cellulose-binding tunnel-enclosing loop motifs, which have been suggested to influence intrinsic cellobiohydrolase properties, such as endo-initiation, processivity, and off-rate. The Tat Cel7A, Tha Cel7A, and Tre Cel7A enzymes were characterized for comparison of function. The catalytic domain of Tat Cel7A was crystallized, and two structures were determined: without ligand and with thio-cellotriose in the active site. Initial hydrolysis of bacterial cellulose was faster with Tat Cel7A than either Tha Cel7A or Tre Cel7A. In synergistic saccharification of pretreated corn stover, both Tat Cel7A and Tha Cel7A were more efficient than Tre Cel7A, although Tat Cel7A was more sensitive to thermal inactivation. Structural analyses and molecular dynamics (MD) simulations were performed to elucidate important structure/function correlations. Moreover, reverse conservation analysis (RCA) of sequence diversity revealed divergent regions of interest located outside the cellulose-binding tunnel of Trichoderma spp. GH7 CBHs. We hypothesize that the combination of loop motifs is the main determinant for the observed differences in Cel7A activity on cellulosic substrates. Fine-tuning of the loop flexibility appears to be an important evolutionary target in Trichoderma spp., a conclusion supported by the RCA data. Our results indicate that, for industrial use, it would be beneficial to combine loop motifs from Tat Cel7A with the thermostability features of Tre Cel7A. Furthermore, one region implicated in thermal unfolding is suggested as a primary target for protein engineering.
Organizational Affiliation:
1Department of Molecular Sciences, Swedish University of Agricultural Sciences, P.O. Box 7015, 750 07 Uppsala, Sweden.