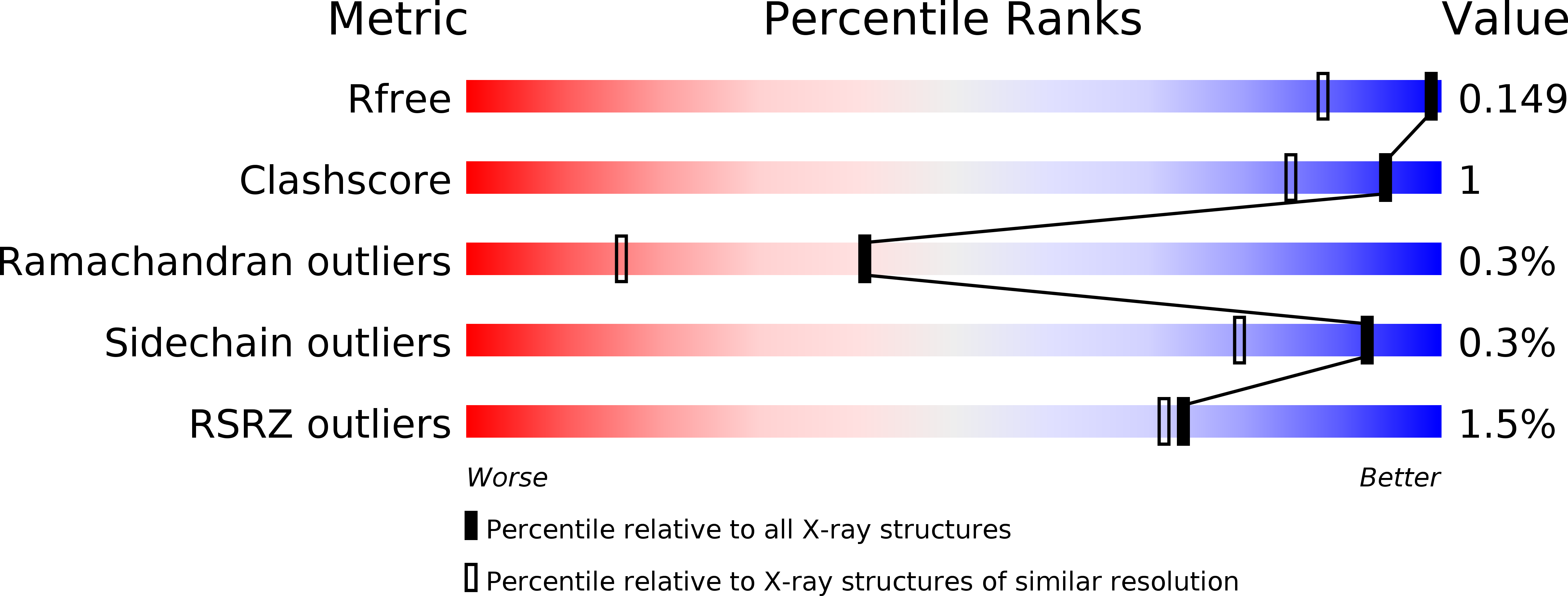
Structures and Mechanisms of the Non-Heme Fe(II)- and 2-Oxoglutarate-Dependent Ethylene-Forming Enzyme: Substrate Binding Creates a Twist.
Martinez, S., Fellner, M., Herr, C.Q., Ritchie, A., Hu, J., Hausinger, R.P.(2017) J Am Chem Soc 139: 11980-11988
- PubMed: 28780854
- DOI: https://doi.org/10.1021/jacs.7b06186
- Primary Citation of Related Structures:
5V2T, 5V2U, 5V2V, 5V2X, 5V2Y, 5V2Z, 5V31, 5V32, 5V34, 5VKA, 5VKB - PubMed Abstract:
The ethylene-forming enzyme (EFE) from Pseudomonas syringae pv. phaseolicola PK2 is a member of the mononuclear nonheme Fe(II)- and 2-oxoglutarate (2OG)-dependent oxygenase superfamily. EFE converts 2OG into ethylene plus three CO 2 molecules while also catalyzing the C5 hydroxylation of l-arginine (l-Arg) driven by the oxidative decarboxylation of 2OG to form succinate and CO 2 . Here we report 11 X-ray crystal structures of EFE that provide insight into the mechanisms of these two reactions. Binding of 2OG in the absence of l-Arg resulted in predominantly monodentate metal coordination, distinct from the typical bidentate metal-binding species observed in other family members. Subsequent addition of l-Arg resulted in compression of the active site, a conformational change of the carboxylate side chain metal ligand to allow for hydrogen bonding with the substrate, and creation of a twisted peptide bond involving this carboxylate and the following tyrosine residue. A reconfiguration of 2OG achieves bidentate metal coordination. The dioxygen binding site is located on the metal face opposite to that facing l-Arg, thus requiring reorientation of the generated ferryl species to catalyze l-Arg hydroxylation. Notably, a phenylalanyl side chain pointing toward the metal may hinder such a ferryl flip and promote ethylene formation. Extensive site-directed mutagenesis studies supported the importance of this phenylalanine and confirmed the essential residues used for substrate binding and catalysis. The structural and functional characterization described here suggests that conversion of 2OG to ethylene, atypical among Fe(II)/2OG oxygenases, is facilitated by the binding of l-Arg which leads to an altered positioning of the carboxylate metal ligand, a resulting twisted peptide bond, and the off-line geometry for dioxygen coordination.
Organizational Affiliation:
Department of Microbiology and Molecular Genetics, Michigan State University , East Lansing, Michigan 48824, United States.