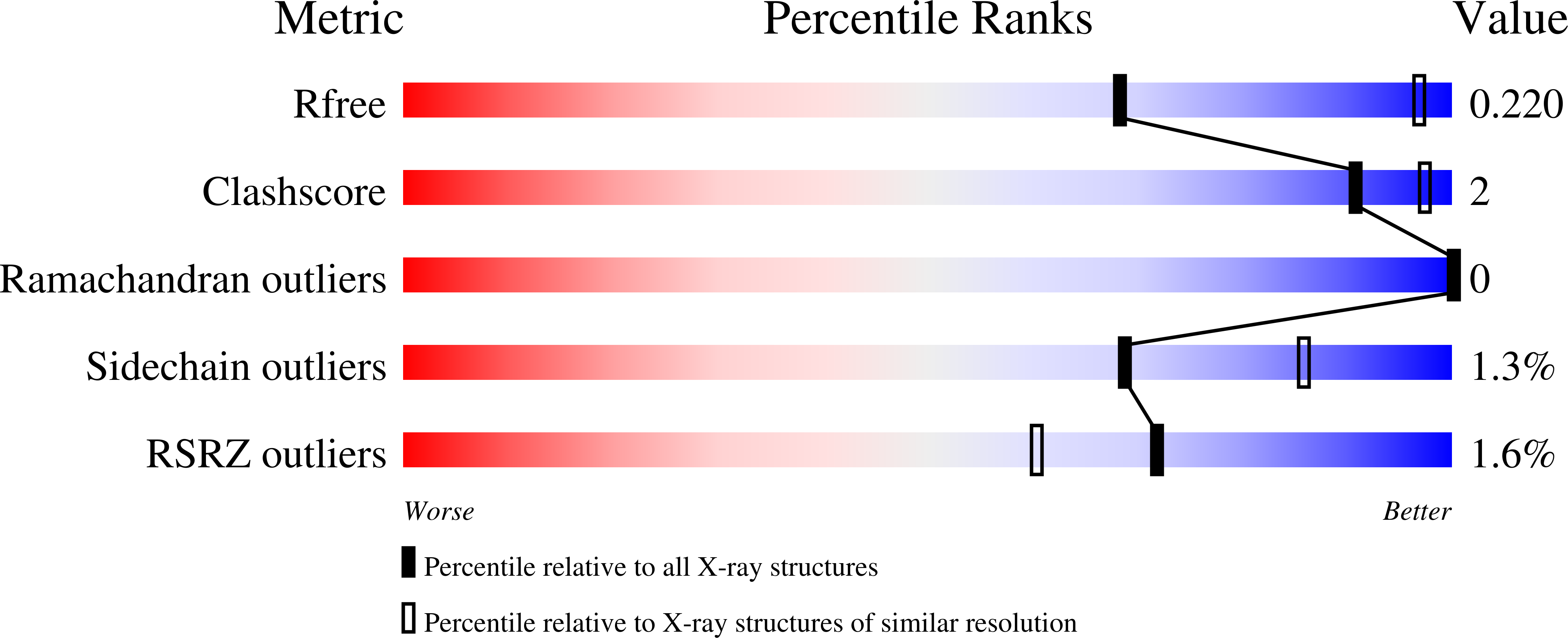
Structural basis for the inhibition of poxvirus assembly by the antibiotic rifampicin.
Garriga, D., Headey, S., Accurso, C., Gunzburg, M., Scanlon, M., Coulibaly, F.(2018) Proc Natl Acad Sci U S A 115: 8424-8429
- PubMed: 30068608
- DOI: https://doi.org/10.1073/pnas.1810398115
- Primary Citation of Related Structures:
6BEB, 6BEC, 6BED, 6BEE, 6BEF, 6BEG, 6BEH, 6BEI - PubMed Abstract:
Poxviruses are large DNA viruses that cause disease in animals and humans. They differ from classical enveloped viruses, because their membrane is acquired from cytoplasmic membrane precursors assembled onto a viral protein scaffold formed by the D13 protein rather than budding through cellular compartments. It was found three decades ago that the antibiotic rifampicin blocks this process and prevents scaffold formation. To elucidate the mechanism of action of rifampicin, we have determined the crystal structures of six D13-rifamycin complexes. These structures reveal that rifamycin compounds bind to a phenylalanine-rich region, or F-ring, at the membrane-proximal opening of the central channel of the D13 trimer. We show by NMR, surface plasmon resonance (SPR), and site-directed mutagenesis that A17, a membrane-associated viral protein, mediates the recruitment of the D13 scaffold by also binding to the F-ring. This interaction is the target of rifampicin, which prevents A17 binding, explaining the inhibition of viral morphogenesis. The F-ring of D13 is both conserved in sequence in mammalian poxviruses and essential for interaction with A17, defining a target for the development of assembly inhibitors. The model of the A17-D13 interaction describes a two-component system for remodeling nascent membranes that may be conserved in other large and giant DNA viruses.
Organizational Affiliation:
Infection and Immunity Program, Biomedicine Discovery Institute, Monash University, Melbourne, VIC 3800, Australia.