Structure-function analysis of sphingamides
Zajonc, D.M., Wang, J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Antigen-presenting glycoprotein CD1d1 | 285 | Mus musculus | Mutation(s): 0 Gene Names: Cd1d1, mCG_3074 | 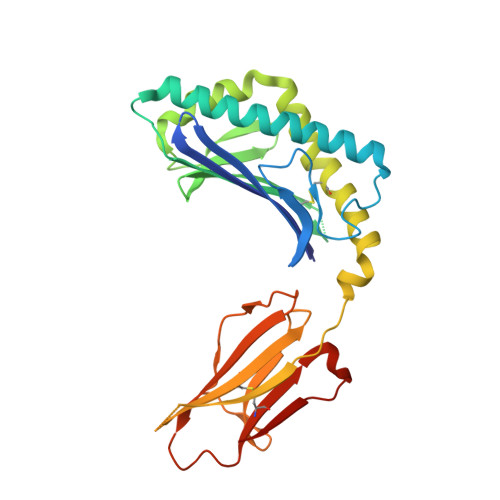 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P11609 (Mus musculus) Explore P11609 Go to UniProtKB: P11609 | |||||
IMPC: MGI:107674 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P11609 | ||||
Glycosylation | |||||
| Glycosylation Sites: 3 | Go to GlyGen: P11609-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Beta-2-microglobulin | 99 | Mus musculus | Mutation(s): 0 Gene Names: B2m | 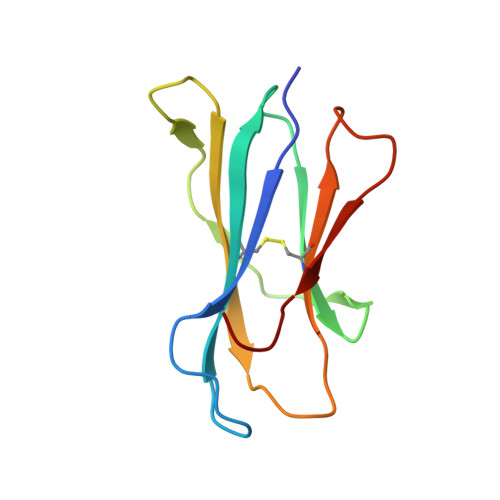 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01887 (Mus musculus) Explore P01887 Go to UniProtKB: P01887 | |||||
IMPC: MGI:88127 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01887 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | C | 6 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G82348BZ GlyCosmos: G82348BZ GlyGen: G82348BZ | |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ELS Query on ELS | F [auth A] | N-[(2S,3S,4R)-3,4-dihydroxy-8-oxo-8-[(4-pentylphenyl)amino]-1-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetr
ahydro-2H-pyran-2-yl]oxy}octan-2-yl]hexacosanamide C51 H92 N2 O10 JRFCMCHAAQOEOJ-BULDXWSMSA-N |  | ||
| NAG Query on NAG | D [auth A], E [auth A] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| NA Query on NA | G [auth A], H [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 41.627 | α = 90 |
| b = 98.476 | β = 106.21 |
| c = 55.431 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |