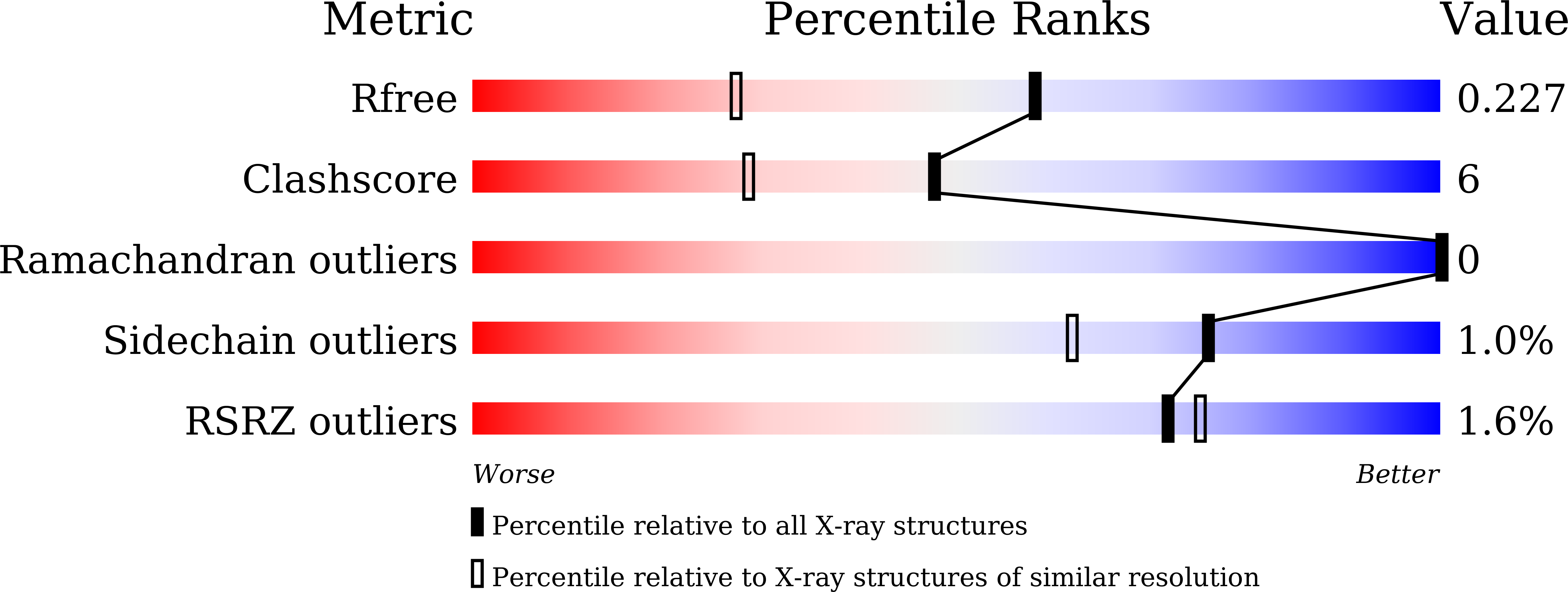
Nylon mesh-based sample holder for fixed-target serial femtosecond crystallography.
Lee, D., Baek, S., Park, J., Lee, K., Kim, J., Lee, S.J., Chung, W.K., Lee, J.L., Cho, Y., Nam, K.H.(2019) Sci Rep 9: 6971-6971
- PubMed: 31061502
- DOI: https://doi.org/10.1038/s41598-019-43485-z
- Primary Citation of Related Structures:
6IRJ, 6IRK - PubMed Abstract:
Fixed-target serial femtosecond crystallography (FT-SFX) was an important advance in crystallography by dramatically reducing sample consumption, while maintaining the benefits of SFX for obtaining crystal structures at room temperature without radiation damage. Despite a number of advantages, preparation of a sample holder for the sample delivery in FT-SFX with the use of many crystals in a single mount at ambient temperature is challenging as it can be complicated and costly, and thus, development of an efficient sample holder is essential. In this study, we introduced a nylon mesh-based sample holder enclosed by a polyimide film. This sample holder can be rapidly manufactured using a commercially available nylon mesh with pores of a desired size at a low cost without challenging technology. Furthermore, this simple device is highly efficient in data acquisition. We performed FT-SFX using a nylon mesh-based sample holder and collected over 130,000 images on a single sample holder using a 30 Hz X-ray pulse for 1.2 h. We determined the crystal structures of lysozyme and glucose isomerase using the nylon mesh at 1.65 and 1.75 Å, respectively. The nylon mesh exposed to X-rays produced very low levels of background scattering at 3.75 and 4.30 Å, which are negligible for data analysis. Our method provides a simple and rapid but highly efficient way to deliver samples for FT-SFX.
Organizational Affiliation:
Department of Mechanical Engineering, POSTECH, Pohang, 37673, Republic of Korea.