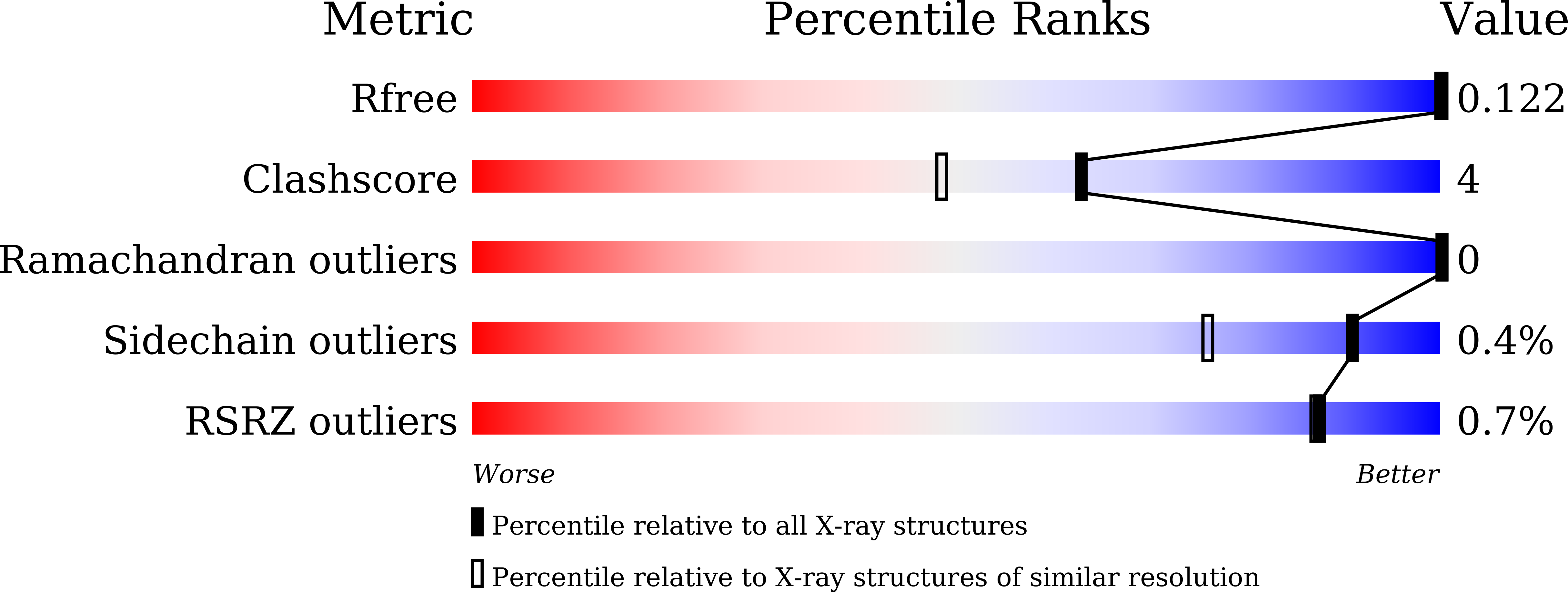
X-ray Crystallography and Electron Paramagnetic Resonance Spectroscopy Reveal Active Site Rearrangement of Cold-Adapted Inorganic Pyrophosphatase.
Horitani, M., Kusubayashi, K., Oshima, K., Yato, A., Sugimoto, H., Watanabe, K.(2020) Sci Rep 10: 4368-4368
- PubMed: 32152422
- DOI: https://doi.org/10.1038/s41598-020-61217-6
- Primary Citation of Related Structures:
6LL7, 6LL8 - PubMed Abstract:
Inorganic pyrophosphatase (PPase) catalyses the hydrolysis reaction of inorganic pyrophosphate to phosphates. Our previous studies showed that manganese (Mn) activated PPase from the psychrophilic bacterium Shewanella sp. AS-11 (Mn-Sh-PPase) has a characteristic temperature dependence of the activity with an optimum at 5 °C. Here we report the X-ray crystallography and electron paramagnetic resonance (EPR) spectroscopy structural analyses of Sh-PPase in the absence and presence of substrate analogues. We successfully determined the crystal structure of Mn-Sh-PPase without substrate and Mg-activated Sh-PPase (Mg-Sh-PPase) complexed with substrate analogue (imidodiphosphate; PNP). Crystallographic studies revealed a bridged water placed at a distance from the di-Mn centre in Mn-Sh-PPase without substrate. The water came closer to the metal centre when PNP bound. EPR analysis of Mn-Sh-PPase without substrate revealed considerably weak exchange coupling, whose magnitude was increased by binding of substrate analogues. The data indicate that the bridged molecule has weak bonds with the di-Mn centre, which suggests a 'loose' structure, whereas it comes closer to di-Mn centre by substrate binding, which suggests a 'well-tuned' structure for catalysis. Thus, we propose that Sh-PPase can rearrange the active site and that the 'loose' structure plays an important role in the cold adaptation mechanism.
Organizational Affiliation:
Department of Applied Biochemistry and Food Science, Saga University, 1 Honjo-machi, Saga, Saga, 840-8502, Japan. [email protected].