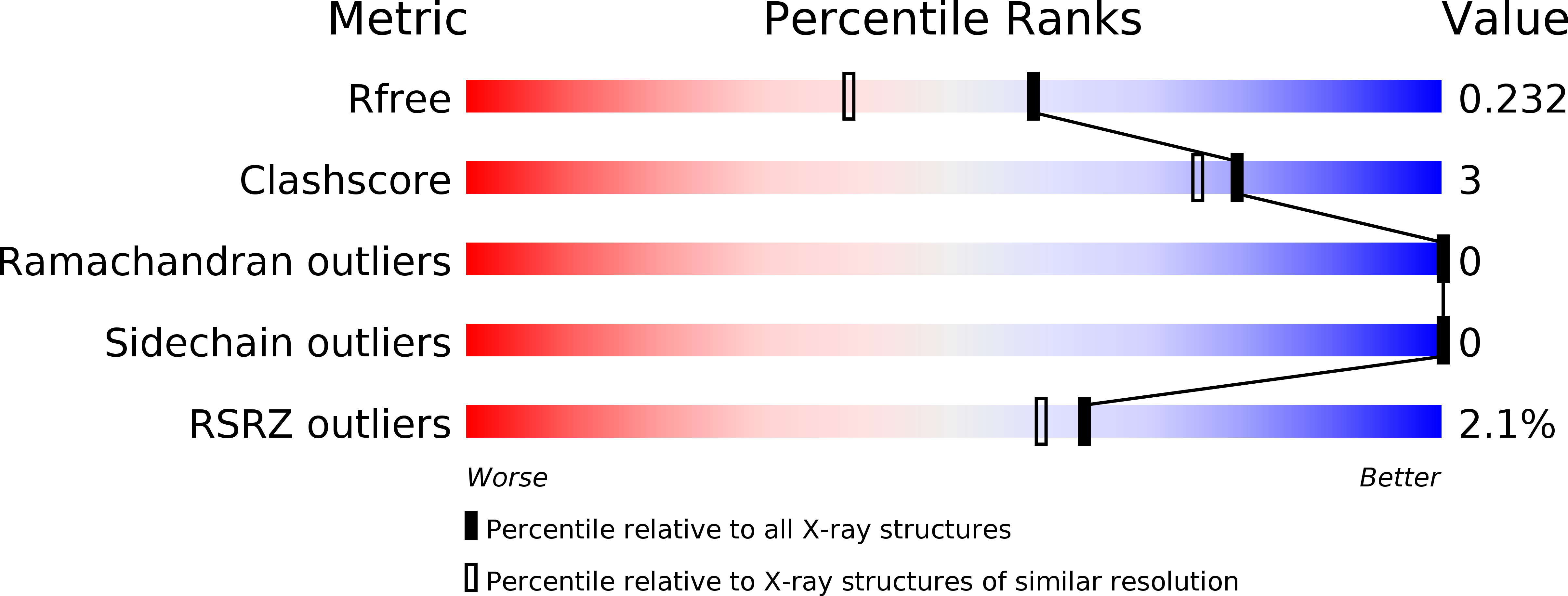
NAD+cleavage activity by animal and plant TIR domains in cell death pathways.
Horsefield, S., Burdett, H., Zhang, X., Manik, M.K., Shi, Y., Chen, J., Qi, T., Gilley, J., Lai, J.S., Rank, M.X., Casey, L.W., Gu, W., Ericsson, D.J., Foley, G., Hughes, R.O., Bosanac, T., von Itzstein, M., Rathjen, J.P., Nanson, J.D., Boden, M., Dry, I.B., Williams, S.J., Staskawicz, B.J., Coleman, M.P., Ve, T., Dodds, P.N., Kobe, B.(2019) Science 365: 793-799
- PubMed: 31439792
- DOI: https://doi.org/10.1126/science.aax1911
- Primary Citation of Related Structures:
6O0Q, 6O0R, 6O0S, 6O0T, 6O0U, 6O0V, 6O0W, 6O1B - PubMed Abstract:
SARM1 (sterile alpha and TIR motif containing 1) is responsible for depletion of nicotinamide adenine dinucleotide in its oxidized form (NAD + ) during Wallerian degeneration associated with neuropathies. Plant nucleotide-binding leucine-rich repeat (NLR) immune receptors recognize pathogen effector proteins and trigger localized cell death to restrict pathogen infection. Both processes depend on closely related Toll/interleukin-1 receptor (TIR) domains in these proteins, which, as we show, feature self-association-dependent NAD + cleavage activity associated with cell death signaling. We further show that SARM1 SAM (sterile alpha motif) domains form an octamer essential for axon degeneration that contributes to TIR domain enzymatic activity. The crystal structures of ribose and NADP + (the oxidized form of nicotinamide adenine dinucleotide phosphate) complexes of SARM1 and plant NLR RUN1 TIR domains, respectively, reveal a conserved substrate binding site. NAD + cleavage by TIR domains is therefore a conserved feature of animal and plant cell death signaling pathways.
Organizational Affiliation:
School of Chemistry and Molecular Biosciences, Institute for Molecular Bioscience and Australian Infectious Diseases Research Centre, University of Queensland, Brisbane, QLD 4072, Australia.