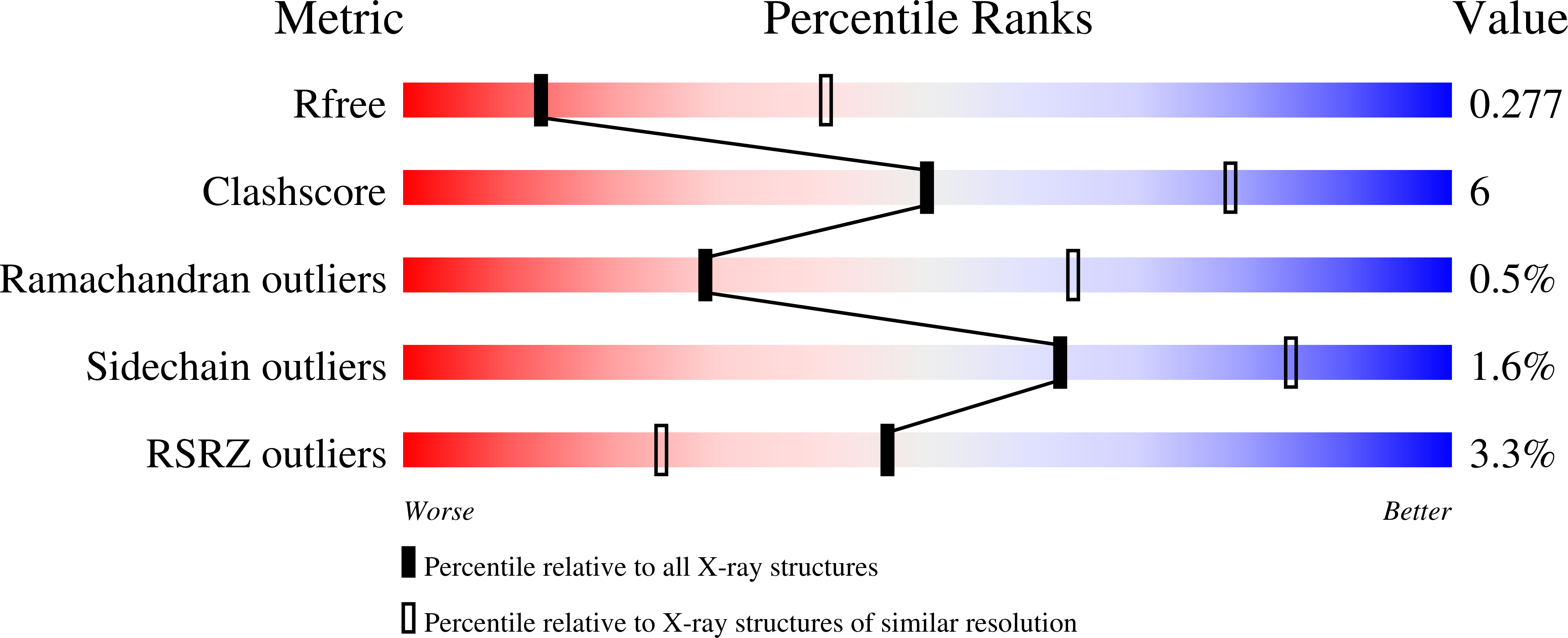
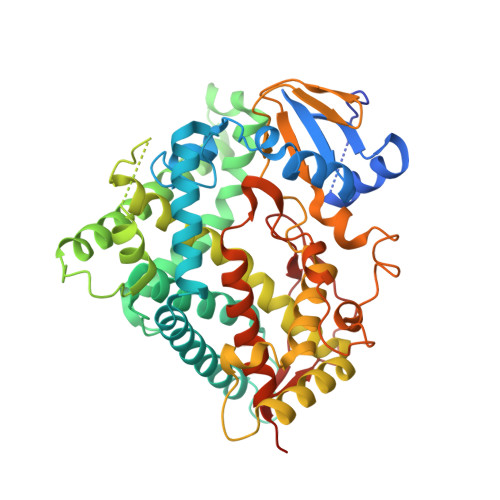
Structure of an ancestral mammalian family 1B1 cytochrome P450 with increased thermostability.
Bart, A.G., Harris, K.L., Gillam, E.M.J., Scott, E.E.(2020) J Biol Chem 295: 5640-5653
- PubMed: 32156703
- DOI: https://doi.org/10.1074/jbc.RA119.010727
- Primary Citation of Related Structures:
6OYU, 6OYV - PubMed Abstract:
Mammalian cytochrome P450 enzymes often metabolize many pharmaceuticals and other xenobiotics, a feature that is valuable in a biotechnology setting. However, extant P450 enzymes are typically relatively unstable, with T 50 values of ∼30-40 °C. Reconstructed ancestral cytochrome P450 enzymes tend to have variable substrate selectivity compared with related extant forms, but they also have higher thermostability and therefore may be excellent tools for commercial biosynthesis of important intermediates, final drug molecules, or drug metabolites. The mammalian ancestor of the cytochrome P450 1B subfamily was herein characterized structurally and functionally, revealing differences from the extant human CYP1B1 in ligand binding, metabolism, and potential molecular contributors to its thermostability. Whereas extant human CYP1B1 has one molecule of α-naphthoflavone in a closed active site, we observed that subtle amino acid substitutions outside the active site in the ancestor CYP1B enzyme yielded an open active site with four ligand copies. A structure of the ancestor with 17β-estradiol revealed only one molecule in the active site, which still had the same open conformation. Detailed comparisons between the extant and ancestor forms revealed increases in electrostatic and aromatic interactions between distinct secondary structure elements in the ancestral forms that may contribute to their thermostability. To the best of our knowledge, this represents the first structural evaluation of a reconstructed ancestral cytochrome P450, revealing key features that appear to contribute to its thermostability.
Organizational Affiliation:
Program in Biophysics, University of Michigan, Ann Arbor, Michigan 48109.