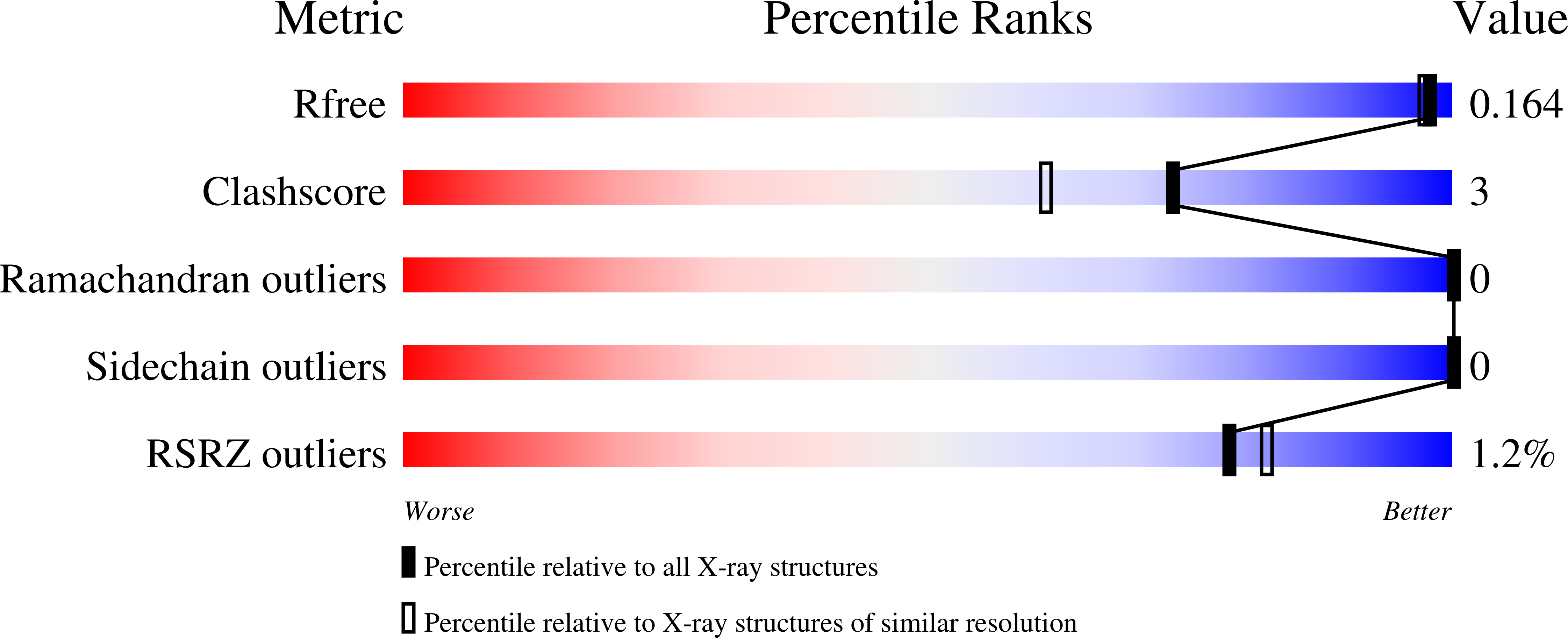
Small-molecule covalent bond formation at tyrosine creates a binding site and inhibits activation of Ral GTPases.
Bum-Erdene, K., Liu, D., Gonzalez-Gutierrez, G., Ghozayel, M.K., Xu, D., Meroueh, S.O.(2020) Proc Natl Acad Sci U S A 117: 7131-7139
- PubMed: 32179690
- DOI: https://doi.org/10.1073/pnas.1913654117
- Primary Citation of Related Structures:
6P0I, 6P0J, 6P0K, 6P0L, 6P0M, 6P0N, 6P0O - PubMed Abstract:
Ral (Ras-like) GTPases are directly activated by oncogenic Ras GTPases. Mutant K-Ras (G12C) has enabled the development of covalent K-Ras inhibitors currently in clinical trials. However, Ral, and the overwhelming majority of mutant oncogenic K-Ras, are devoid of a druggable pocket and lack an accessible cysteine for the development of a covalent inhibitor. Here, we report that covalent bond formation by an aryl sulfonyl fluoride electrophile at a tyrosine residue (Tyr-82) inhibits guanine exchange factor Rgl2-mediated nucleotide exchange of Ral GTPase. A high-resolution 1.18-Å X-ray cocrystal structure shows that the compound binds to a well-defined binding site in RalA as a result of a switch II loop conformational change. The structure, along with additional high-resolution crystal structures of several analogs in complex with RalA, confirm the importance of key hydrogen bond anchors between compound sulfone oxygen atoms and Ral backbone nitrogen atoms. Our discovery of a pocket with features found on known druggable sites and covalent modification of a bystander tyrosine residue present in Ral and Ras GTPases provide a strategy that could lead to therapeutic agent targeting oncogenic Ras mutants that are devoid of a cysteine nucleophile.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN 46202.