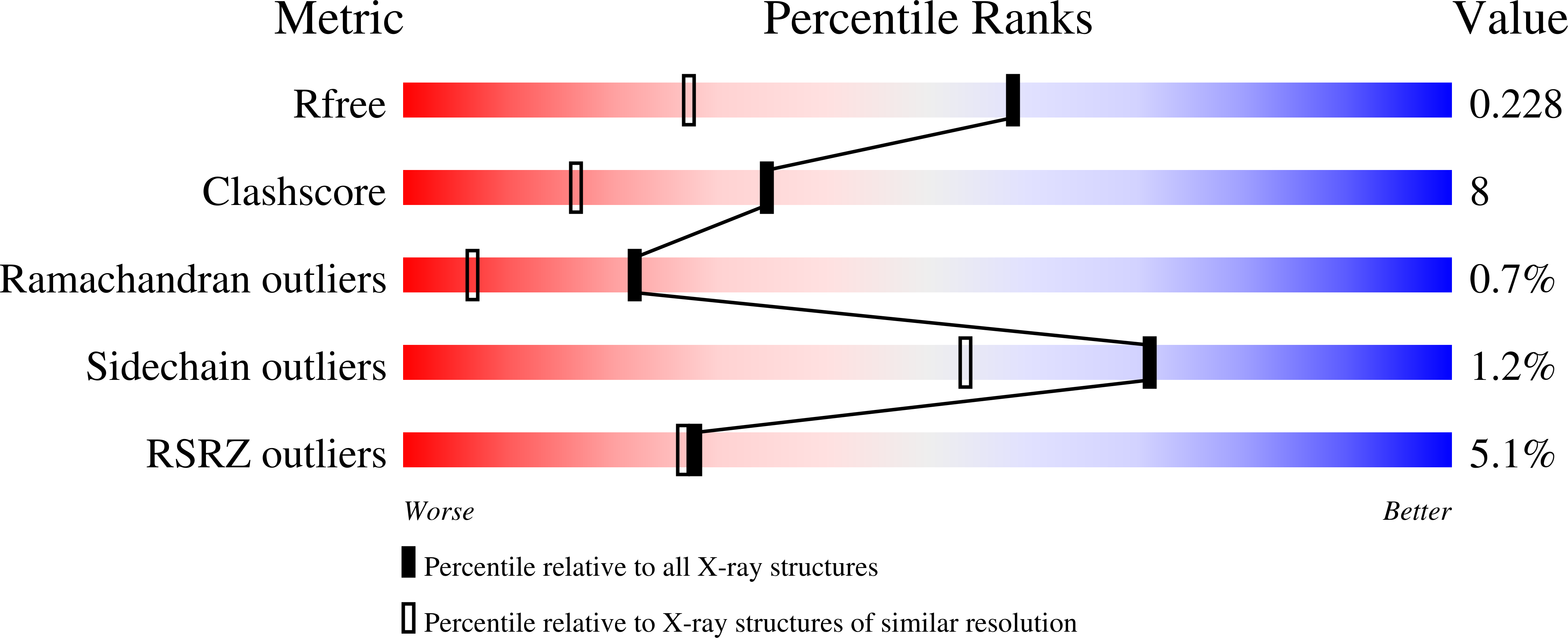
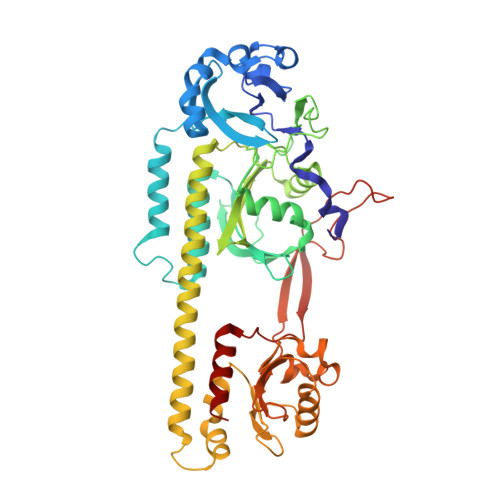
High-resolution crystal structures of a myxobacterial phytochrome at cryo and room temperatures.
Sanchez, J.C., Carrillo, M., Pandey, S., Noda, M., Aldama, L., Feliz, D., Claesson, E., Wahlgren, W.Y., Tracy, G., Duong, P., Nugent, A.C., Field, A., Srajer, V., Kupitz, C., Iwata, S., Nango, E., Tanaka, R., Tanaka, T., Fangjia, L., Tono, K., Owada, S., Westenhoff, S., Schmidt, M., Stojkovic, E.A.(2019) Struct Dyn 6: 054701-054701
- PubMed: 31559319
- DOI: https://doi.org/10.1063/1.5120527
- Primary Citation of Related Structures:
6PTQ, 6PTX, 6PU2 - PubMed Abstract:
Phytochromes (PHYs) are photoreceptor proteins first discovered in plants, where they control a variety of photomorphogenesis events. PHYs as photochromic proteins can reversibly switch between two distinct states: a red light (Pr) and a far-red light (Pfr) absorbing form. The discovery of Bacteriophytochromes (BphPs) in nonphotosynthetic bacteria has opened new frontiers in our understanding of the mechanisms by which these natural photoswitches can control single cell development, although the role of BphPs in vivo remains largely unknown. BphPs are dimeric proteins that consist of a photosensory core module (PCM) and an enzymatic domain, often a histidine kinase. The PCM is composed of three domains (PAS, GAF, and PHY). It holds a covalently bound open-chain tetrapyrrole (biliverdin, BV) chromophore. Upon absorption of light, the double bond between BV rings C and D isomerizes and reversibly switches the protein between Pr and Pfr states. We report crystal structures of the wild-type and mutant (His275Thr) forms of the canonical BphP from the nonphotosynthetic myxobacterium Stigmatella aurantiaca ( Sa BphP2) in the Pr state. Structures were determined at 1.65 Å and 2.2 Å (respectively), the highest resolution of any PCM construct to date. We also report the room temperature wild-type structure of the same protein determined at 2.1 Å at the SPring-8 Angstrom Compact free electron LAser (SACLA), Japan. Our results not only highlight and confirm important amino acids near the chromophore that play a role in Pr-Pfr photoconversion but also describe the signal transduction into the PHY domain which moves across tens of angstroms after the light stimulus.
Organizational Affiliation:
Department of Biology, Northeastern Illinois University, 5500 N. St. Louis Ave., Chicago, Illinois 60625, USA.