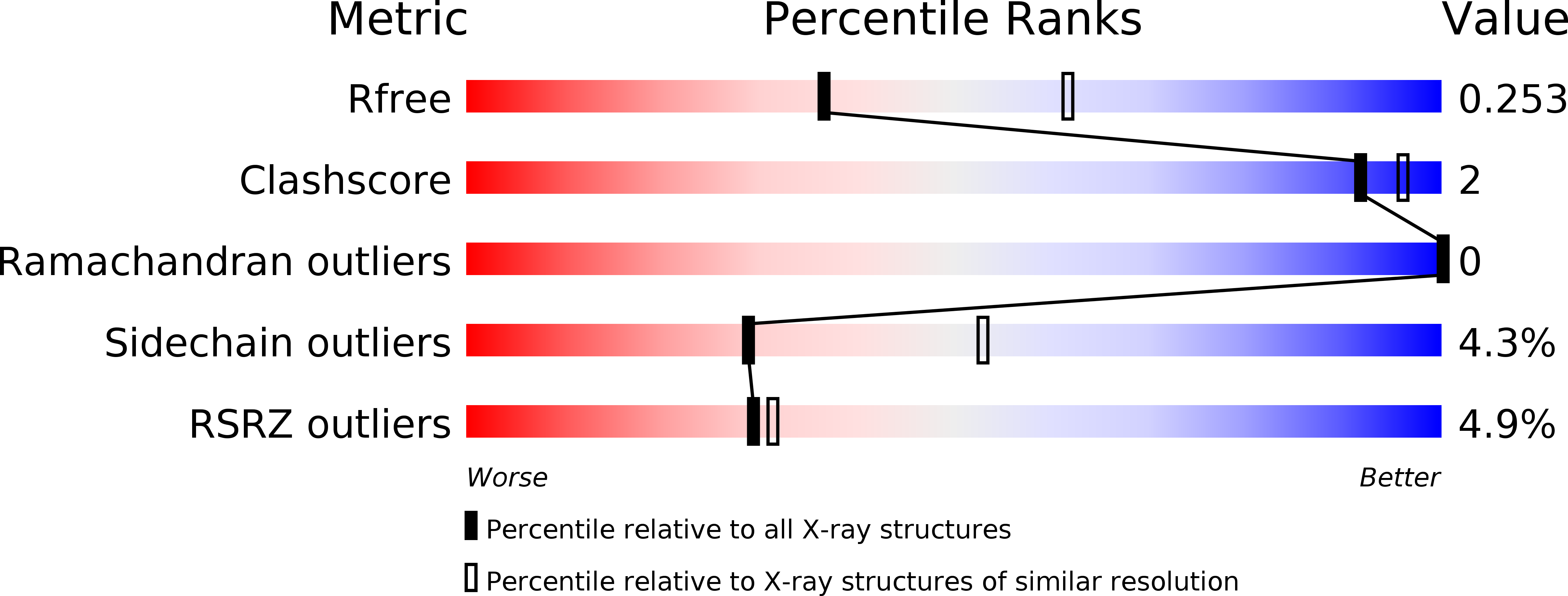
Structural and Molecular Insight into Resistance Mechanisms of First Generation cMET Inhibitors.
Collie, G.W., Koh, C.M., O'Neill, D.J., Stubbs, C.J., Khurana, P., Eddershaw, A., Snijder, A., Mauritzson, F., Barlind, L., Dale, I.L., Shaw, J., Phillips, C., Hennessy, E.J., Cheung, T., Narvaez, A.J.(2019) ACS Med Chem Lett 10: 1322-1327
- PubMed: 31531204
- DOI: https://doi.org/10.1021/acsmedchemlett.9b00276
- Primary Citation of Related Structures:
6SD9, 6SDC, 6SDD, 6SDE - PubMed Abstract:
Many small molecule inhibitors of the cMET receptor tyrosine kinase have been evaluated in clinical trials for the treatment of cancer and resistance-conferring mutations of cMET are beginning to be reported for a number of such compounds. There is now a need to understand specific cMET mutations at the molecular level, particularly concerning small molecule recognition. Toward this end, we report here the first crystal structures of the recent clinically observed resistance-conferring D1228V cMET mutant in complex with small molecule inhibitors, along with a crystal structure of wild-type cMET bound by the clinical compound savolitinib and supporting cellular, biochemical, and biophysical data. Our findings indicate that the D1228V alteration induces conformational changes in the kinase, which could have implications for small molecule inhibitor design. The data we report here increases our molecular understanding of the D1228V cMET mutation and provides insight for future inhibitor design.
Organizational Affiliation:
Discovery Sciences, R&D, AstraZeneca, Cambridge, U.K.