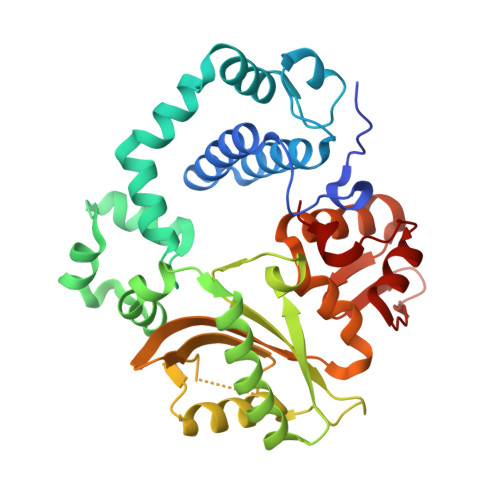
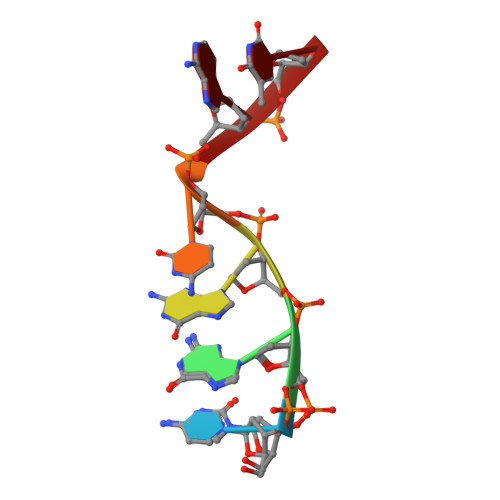
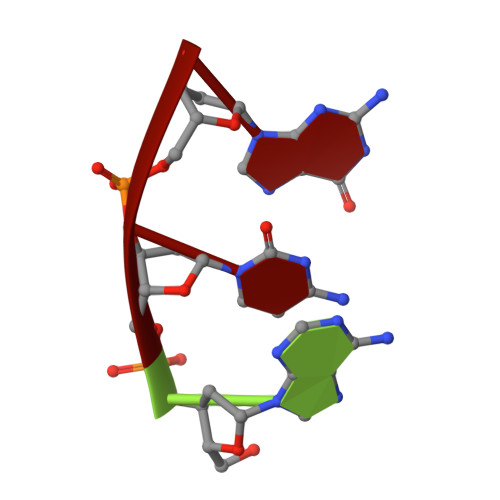
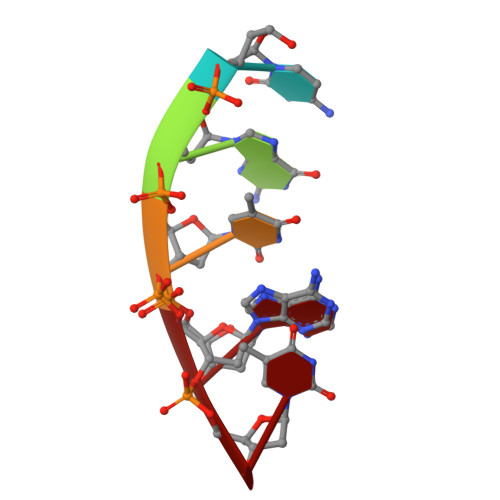
Structural snapshots of human DNA polymerase mu engaged on a DNA double-strand break.
Kaminski, A.M., Pryor, J.M., Ramsden, D.A., Kunkel, T.A., Pedersen, L.C., Bebenek, K.(2020) Nat Commun 11: 4784-4784
- PubMed: 32963245
- DOI: https://doi.org/10.1038/s41467-020-18506-5
- Primary Citation of Related Structures:
6WIC, 6WID, 6WIE - PubMed Abstract:
Genomic integrity is threatened by cytotoxic DNA double-strand breaks (DSBs), which must be resolved efficiently to prevent sequence loss, chromosomal rearrangements/translocations, or cell death. Polymerase μ (Polμ) participates in DSB repair via the nonhomologous end-joining (NHEJ) pathway, by filling small sequence gaps in broken ends to create substrates ultimately ligatable by DNA Ligase IV. Here we present structures of human Polμ engaging a DSB substrate. Synapsis is mediated solely by Polμ, facilitated by single-nucleotide homology at the break site, wherein both ends of the discontinuous template strand are stabilized by a hydrogen bonding network. The active site in the quaternary Pol μ complex is poised for catalysis and nucleotide incoporation proceeds in crystallo. These structures demonstrate that Polμ may address complementary DSB substrates during NHEJ in a manner indistinguishable from single-strand breaks.
Organizational Affiliation:
Genome Integrity and Structural Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, 111 TW Alexander Dr., Bldg. 101/Rm F338, Research Triangle Park, NC, 27709, USA.