Single nucleotide variants lead to dysregulation of the human mitochondrial NAD(P) + -dependent malic enzyme.
Hsieh, J.Y., Yang, H.P., Tewary, S.K., Cheng, H.C., Liu, Y.L., Tai, S.C., Chen, W.L., Hsu, C.H., Huang, T.J., Chou, C.J., Huang, Y.N., Peng, C.T., Ho, M.C., Liu, G.Y., Hung, H.C.(2021) iScience 24: 102034-102034
- PubMed: 33554057
- DOI: https://doi.org/10.1016/j.isci.2021.102034
- Primary Citation of Related Structures:
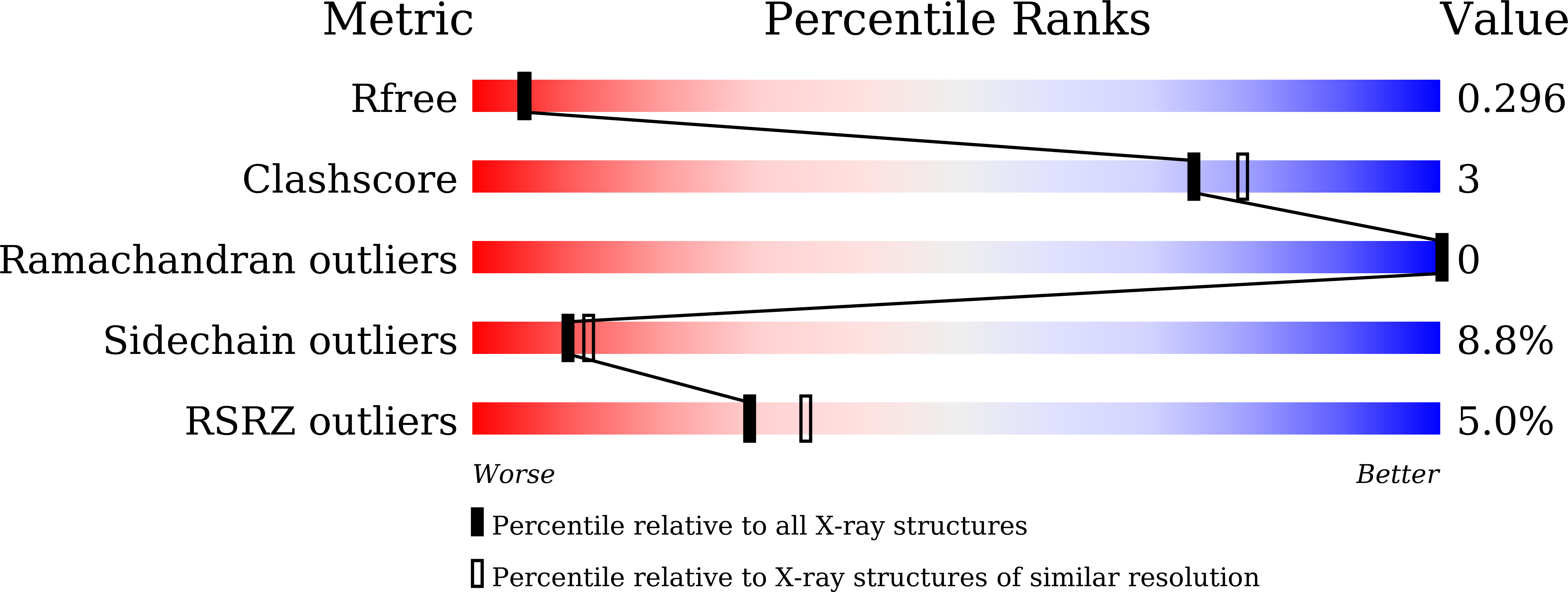
7BSJ, 7BSK, 7BSL - PubMed Abstract:
Human mitochondrial NAD(P) + -dependent malic enzyme (ME2) is well recognized to associate with cancer cell metabolism, and the single nucleotide variants (SNVs) of ME2 may play a role in enzyme regulation. Here we reported that the SNVs of ME2 occurring in the allosteric sites lead to inactivation or overactivation of ME2. Two ME2-SNVs, ME2_R67Q and ME2-R484W, that demonstrated inactivating or overactivating enzyme activities of ME2, respectively, have different impact toward the cells. The cells with overactivating SNV enzyme, ME2_R484W, grow more rapidly and are more resistant to cellular senescence than the cells with wild-type or inactivating SNV enzyme, ME2_R67Q. Crystal structures of these two ME2-SNVs reveal that ME2_R67Q was an inactivating "dead form," and ME2_R484W was an overactivating "closed form" of the enzyme. The resolved ME2-SNV structures provide a molecular basis to explain the abnormal kinetic properties of these SNV enzymes.
Organizational Affiliation:
Department of Life Sciences, National Chung Hsing University, Taichung, Taiwan.