Androstenedione-bound structure of CYP154C2 from Streptomyces avermitilis in an open conformation
Xu, L.H., Fushinobu, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytochrome P450 hydroxylase | 400 | Streptomyces avermitilis MA-4680 = NBRC 14893 | Mutation(s): 0 Gene Names: cyp19, SAVERM_3882 | 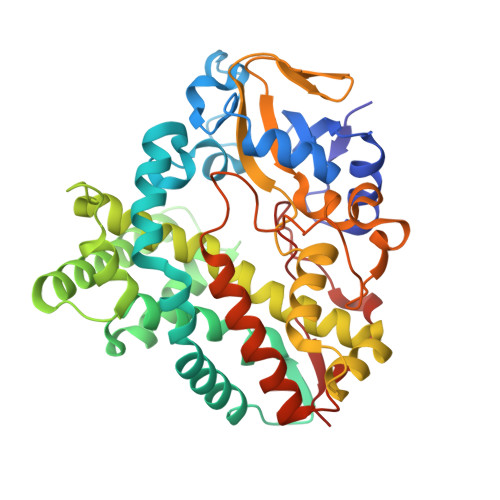 | |
UniProt | |||||
Find proteins for Q82GL5 (Streptomyces avermitilis (strain ATCC 31267 / DSM 46492 / JCM 5070 / NBRC 14893 / NCIMB 12804 / NRRL 8165 / MA-4680)) Explore Q82GL5 Go to UniProtKB: Q82GL5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q82GL5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HEM Query on HEM | B [auth A] | PROTOPORPHYRIN IX CONTAINING FE C34 H32 Fe N4 O4 KABFMIBPWCXCRK-RGGAHWMASA-L |  | ||
| ASD Query on ASD | C [auth A] | 4-ANDROSTENE-3-17-DIONE C19 H26 O2 AEMFNILZOJDQLW-QAGGRKNESA-N |  | ||
| PEG Query on PEG | D [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 54.6 | α = 90 |
| b = 80.13 | β = 90 |
| c = 120.63 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| iMOSFLM | data reduction |
| SCALA | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 81402810 |