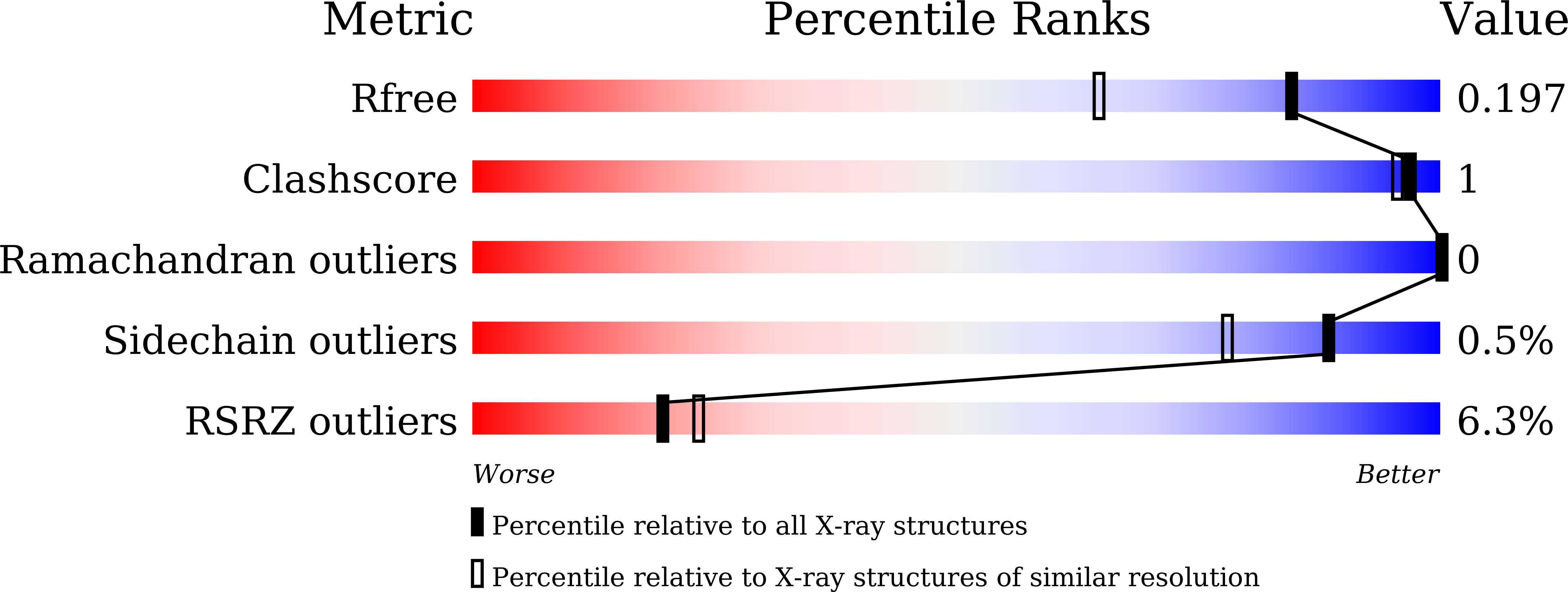
Identification of the first highly selective inhibitor of human lactate dehydrogenase B.
Shibata, S., Sogabe, S., Miwa, M., Fujimoto, T., Takakura, N., Naotsuka, A., Kitamura, S., Kawamoto, T., Soga, T.(2021) Sci Rep 11: 21353-21353
- PubMed: 34725423
- DOI: https://doi.org/10.1038/s41598-021-00820-7
- Primary Citation of Related Structures:
7DBJ, 7DBK - PubMed Abstract:
Lactate dehydrogenase (LDH) catalyses the conversion of pyruvate to lactate and NADH to NAD + ; it has two isoforms, LDHA and LDHB. LDHA is a promising target for cancer therapy, whereas LDHB is necessary for basal autophagy and cancer cell proliferation in oxidative and glycolytic cancer cells. To the best of our knowledge, selective inhibitors for LDHB have not yet been reported. Here, we developed a high-throughput mass spectrometry screening system using an LDHB enzyme assay by detecting NADH and NAD + . As a result, we identified a small-molecule LDHB selective inhibitor AXKO-0046, an indole derivative. This compound exhibited uncompetitive LDHB inhibition (EC 50 = 42 nM). X-ray crystallography revealed that AXKO-0046 bound to the potential allosteric site away from the LDHB catalytic active site, suggesting that targeting the tetramerisation interface of the two dimers is critical for the enzymatic activity. AXKO-0046 and its derivatives can be used to validate LDHB-associated pathways in cancer metabolism.
Organizational Affiliation:
Discovery Biology, Discovery Science, Axcelead Drug Discovery Partners, Inc., 2-26-1 Muraoka-Higashi, Fujisawa, Kanagawa, Japan. [email protected].