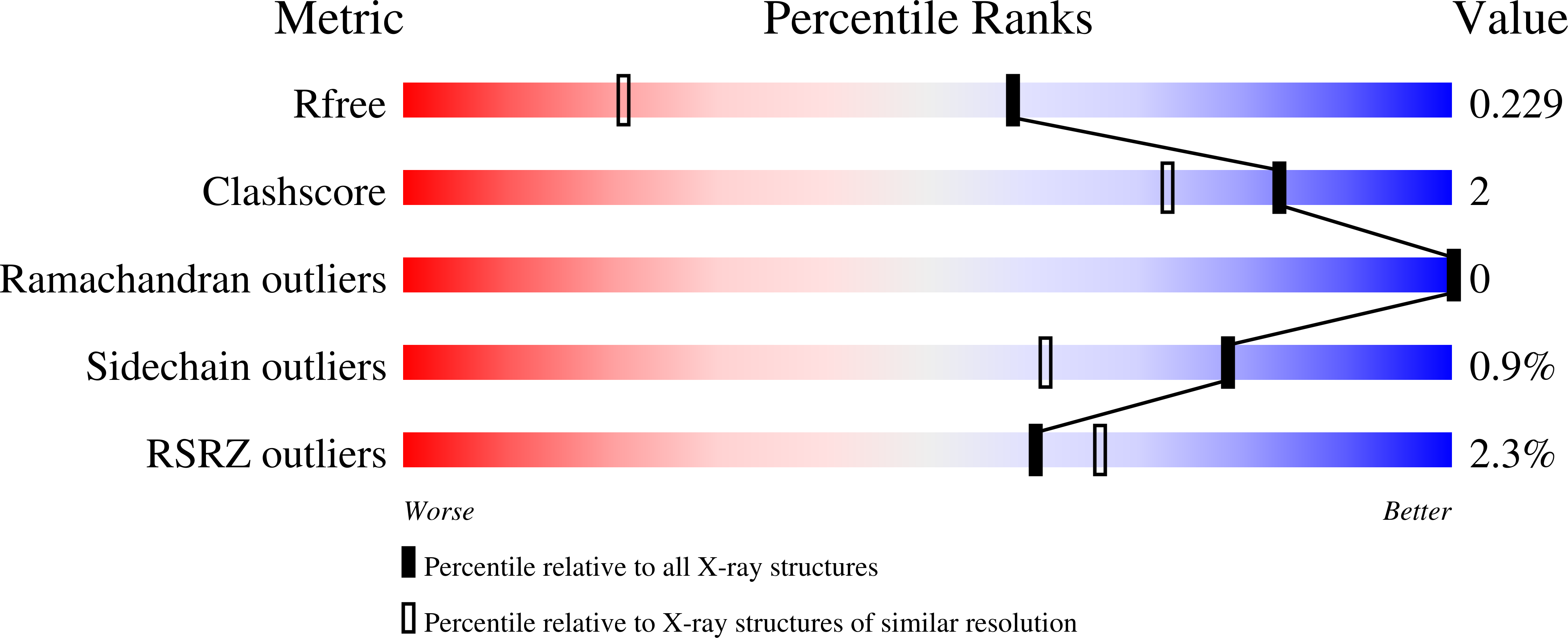
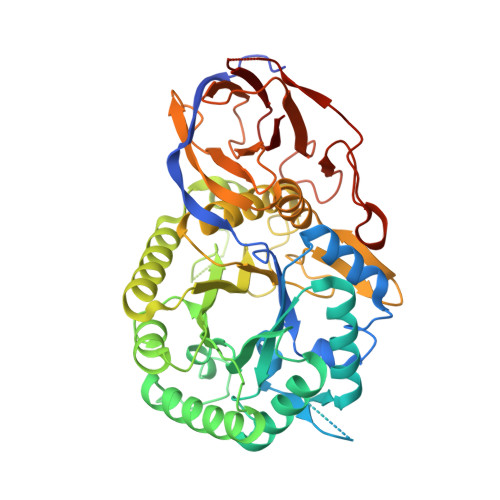
Mechanism-based heparanase inhibitors reduce cancer metastasis in vivo.
de Boer, C., Armstrong, Z., Lit, V.A.J., Barash, U., Ruijgrok, G., Boyango, I., Weitzenberg, M.M., Schroder, S.P., Sarris, A.J.C., Meeuwenoord, N.J., Bule, P., Kayal, Y., Ilan, N., Codee, J.D.C., Vlodavsky, I., Overkleeft, H.S., Davies, G.J., Wu, L.(2022) Proc Natl Acad Sci U S A 119: e2203167119-e2203167119
- PubMed: 35881786
- DOI: https://doi.org/10.1073/pnas.2203167119
- Primary Citation of Related Structures:
7PR6, 7PR7, 7PR8, 7PR9, 7PRB, 7PRT, 7PSH, 7PSI, 7PSJ, 7PSK - PubMed Abstract:
Heparan sulfate proteoglycans (HSPGs) mediate essential interactions throughout the extracellular matrix (ECM), providing signals that regulate cellular growth and development. Altered HSPG composition during tumorigenesis strongly aids cancer progression. Heparanase (HPSE) is the principal enzyme responsible for extracellular heparan sulfate catabolism and is markedly up-regulated in aggressive cancers. HPSE overactivity degrades HSPGs within the ECM, facilitating metastatic dissemination and releasing mitogens that drive cellular proliferation. Reducing extracellular HPSE activity reduces cancer growth, but few effective inhibitors are known, and none are clinically approved. Inspired by the natural glycosidase inhibitor cyclophellitol, we developed nanomolar mechanism-based, irreversible HPSE inhibitors that are effective within physiological environments. Application of cyclophellitol-derived HPSE inhibitors reduces cancer aggression in cellulo and significantly ameliorates murine metastasis. Mechanism-based irreversible HPSE inhibition is an unexplored anticancer strategy. We demonstrate the feasibility of such compounds to control pathological HPSE-driven malignancies.
Organizational Affiliation:
Department of Bio-organic Synthesis, Leiden Institute of Chemistry, Leiden University, 2333 CC Leiden, The Netherlands.