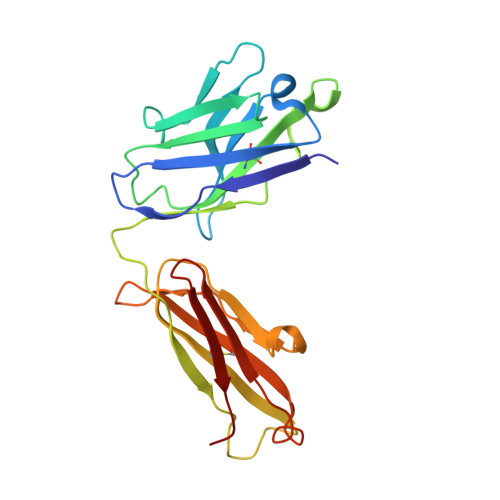
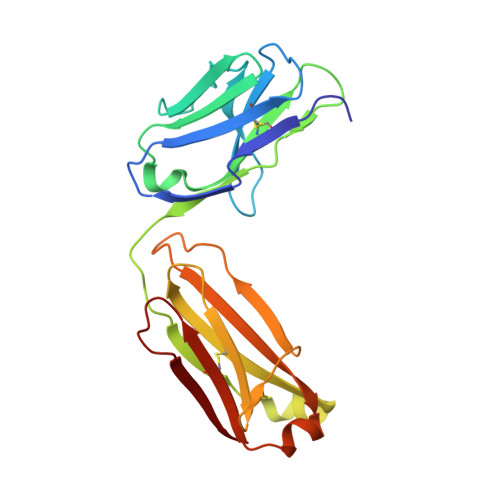
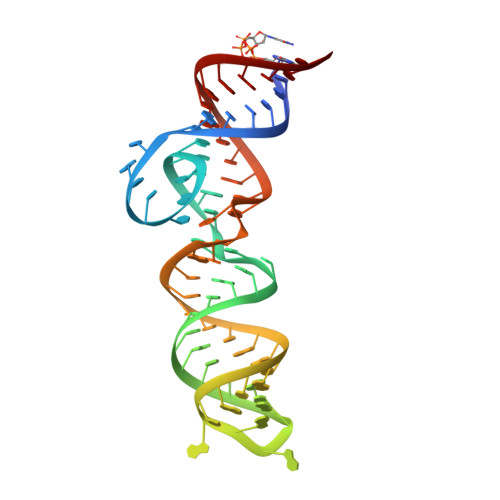
Structural Basis for Fluorescence Activation by Pepper RNA.
Rees, H.C., Gogacz, W., Li, N.S., Koirala, D., Piccirilli, J.A.(2022) ACS Chem Biol 17: 1866-1875
- PubMed: 35759696
- DOI: https://doi.org/10.1021/acschembio.2c00290
- Primary Citation of Related Structures:
7SZU, 7U0Y - PubMed Abstract:
Pepper is a fluorogenic RNA aptamer tag that binds to a variety of benzylidene-cyanophenyl (HBC) derivatives with tight affinity and activates their fluorescence. To investigate how Pepper RNA folds to create a binding site for HBC, we used antibody-assisted crystallography to determine the structures of Pepper bound to HBC530 and HBC599 to 2.3 and 2.7 Å resolutions, respectively. The structural data show that Pepper folds into an elongated structure and organizes nucleotides within an internal bulge to create the ligand binding site, assisted by an out-of-plane platform created by tertiary interactions with an adjacent bulge. As predicted from a lack of K + dependence, Pepper does not use a G-quadruplex to form a binding pocket for HBC. Instead, Pepper uses a unique base-quadruple·base-triple stack to sandwich the ligand with a U·G wobble pair. Site-bound Mg 2+ ions support ligand binding structurally and energetically. This research provides insight into the structural features that allow the Pepper aptamer to bind HBC and show how Pepper's function may expand to allow the in vivo detection of other small molecules and metals.
Organizational Affiliation:
Department of Chemistry, University of Chicago, Chicago, Illinois 60637, United States.